Researchers at the University of Queensland (UG) have published a new paper exploring 3D printing’s role in the future of personalized medicine for patients.
According to pharmacist, UQ PhD student and lead author of the study Liam Krueger, the technology is refined enough to accurately print specialized dosages onsite in hospitals and pharmacies in coming years. Through the study, the researchers are hoping to accelerate the advancement of 3D printed pharmaceuticals within Australia and beyond.
“3D printing is regularly used in other medical settings such as dentistry to create implants, however the utilization of the technology is lagging in the pharmaceutical space,” said Krueger.
“With this research we are hoping to gain more momentum for the implementation of this technology which would be an incredible opportunity for the future of the Australian pharmaceutical landscape.”

3D printing’s pharmaceutical progess
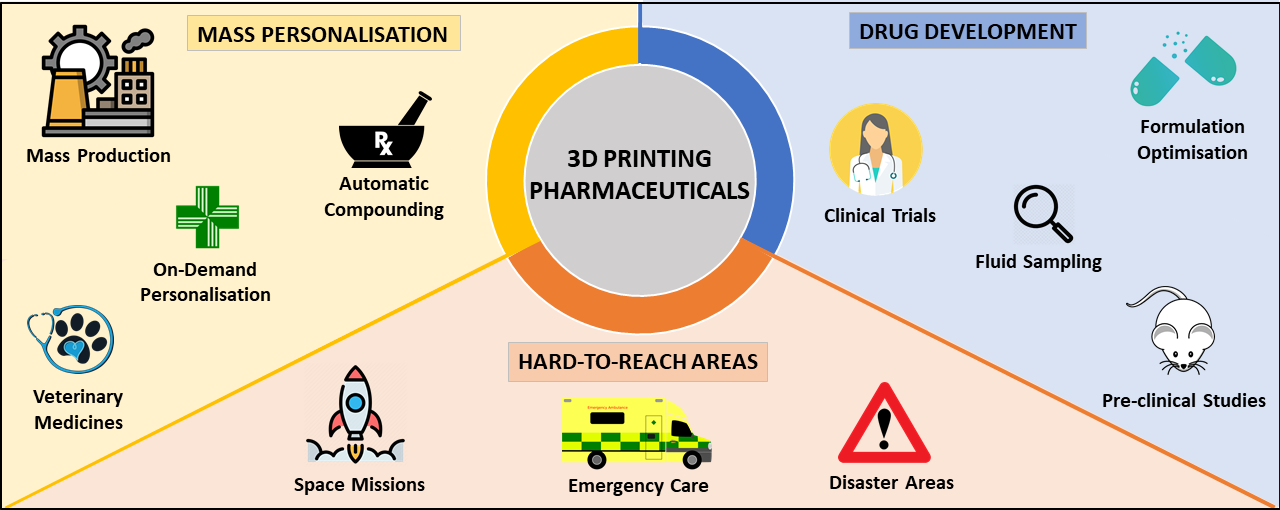
While it has been widely acknowledged that additive manufacturing offers several significant advantages for clinical pharmaceutical drug development, such as personalizing medicines to patient needs, expediting drug delivery times, and providing on-demand treatment, the technology’s transition from lab to the clinic is still largely in its infancy.
There have been some notable developments in this area, though, not least by Aprecia which received the first FDA approval for a 3D printed drug back in 2015. The firm’s Spritam drug was approved for the treatment of epilepsy, and since then Aprecia has continued to scale up its manufacturing output through partnerships with the likes of R&D firm Battelle.
Other players at the forefront of this sector include pharmaceutical 3D printing specialist FabRx, which through the development of its own flexible drug manufacturing platform has developed personalized medicine for the treatment of a rare metabolic disease in children and fabricated its 3D printed “Printlets” tablets with Braille and Moon patterns to aid medicine taking for patient’s with visual impairment.
Elsewhere, global pharmaceutical firm Merck carried out a joint project with EOS Group company AMCM to develop and produce 3D printed tablets in 2020, first for clinical trials and the later for commercial manufacturing.
3D printed pills the future of medicine?
UQ has conducted the latest research in the area of 3D printed pharmaceuticals, with its study suggesting the technology is now refined enough to be deployed in hospitals and pharmacies in the future.
“The advancement of 3D printing technology means that we can tailor medication for patients to ensure it has the exact doses or combinations for their specific needs,” Krueger said. “Through 3D printing we can combine five pills into one, and even change the size, shape, color, flavor, or texture or a pill.”
Despite the clear advantages offered by additive manufacturing in this field, Krueger points out some logistical challenges still need to be addressed before the technology can be adopted on a wider scale within medical settings.
One such challenge is printing time, with the paper predicting a singular, standard-sized 10x3mm pill takes around three minutes to print, while a batch of 28 takes around 45 minutes. Realistically, this is too long a waiting time for the desired on-demand deployment of medicines in clinics, and needs shortening to be realistically applied at scale in real-world settings.
However, the ability of 3D printing to reduce polypharmacy is promising, reasoned Amirali Popat, co-author of the paper and Associate Professor at UQ.
“Polypharmacy is the concurrent use of five or more medicines and roughly two-thirds of Australians aged 75 years are in this classification,” he said. “The real benefit of this technology sits with the consumer or patient, and while we still have a way to go until we see it become a reality in healthcare settings, it’s an extremely exciting development.”
The study is the beginning of a larger body of research UQ is collecting on the viability of using 3D printing for personalized medicine in real-world settings, and so far the data gathered is promising, the researchers said.
According to fellow co-author Chris Freeman, who is also the National President of the Pharmaceutical Society of Australia, “In the future 3D printing could help people with multiple medications in taking the right medication at the right time, at the right dose and potentially reducing nonadherence.”
Further information on the study can be found in the paper titled: “3D printing: Potential clinical applications for personalised solid dose medications,” published in The Medical Journal of Australia. The study is co-authored by L. Krueger, J. Miles, K. Steadman, T. Kumeria, C. Freeman, and A. Popat.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows Spritam, the world’s first FDA-approved 3D printed drug. Photo via Aprecia



