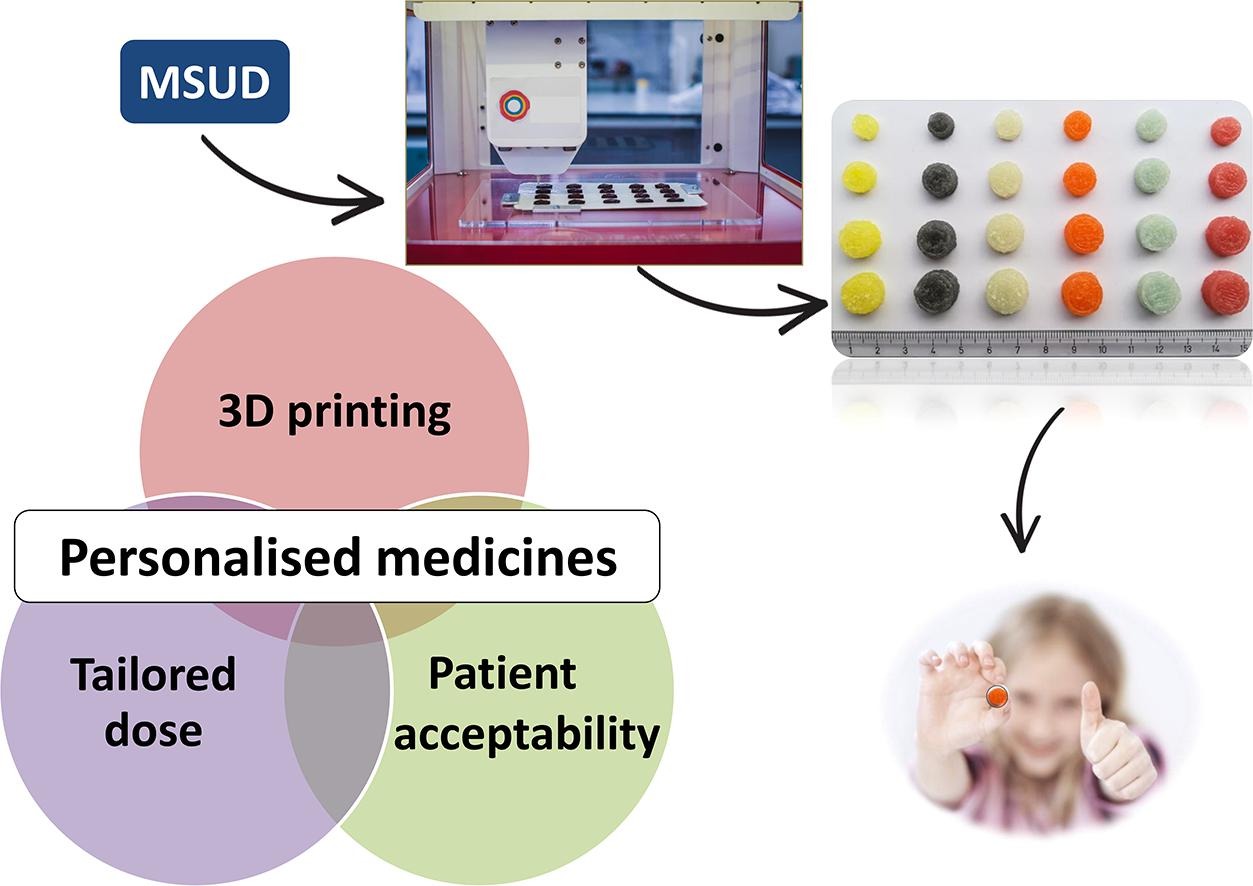
FabRx, a British 3D printed pharmaceuticals company, has developed personalized medicine for children with the rare metabolic disorder, maple syrup urine disease (MSUD).
As a life-long condition, MSUD requires patient-specific therapies and supplements, which are currently provided manually by healthcare providers. FabRx’s 3D printed chewable tablets, called the Printlets, offers a rapid and automated alternative for the preparation of tailored therapeutic dosages.
A human clinical study on the Printlets has been completed between researchers from University College London (UCL), University of Santiago de Compostela (USC), Spain, and the Clinic University Hospital in Santiago de Compostela. Results show that the Printlets have similar effectiveness but higher patient acceptability compared to conventional treatments. Alvaro Goyanes of FabRx said:
“FabRx is delighted to work towards solving problems that are not tackled by the traditional Pharma industry. We are also very glad to collaborate with teams with vision and interest to bring 3D printing of personalized medicines a significant step closer to the clinic”.
3D printing pills
Working at UCL’s Advanced 3D Printing Lab, FabRx has been conducting research surrounding 3D printed medicines for more than 5 years on the principle that everyone is different and needs personal patient care. At present, the company employs four different 3D printing methods (SLA, SLS, FFF and semi-solid extrusion) to medicinal development.
Pill models at the company include its trademark “Printlets” technology – tablets 3D printed to contain specific dosages of a drug in a variety of shapes and sizes, making them easier to swallow. Earlier this year, the company received a £650,000 grant from Innovate UK for the development of a 3D printer adapted for innovative pharmaceutical products.

Maple syrup urine disease
According to Patient UK, MSUD has a worldwide prevalence of 1 in every 185,000 live births. The first line of therapy for MSUD involves a strict diet and amino acid supplements from isoleucine and valine. The dose administered to patients requires strict tailoring according to age, weight and blood concentration of isoleucine.
In current clinical practice, practitioners still have to manually prepare formulations in order to provide patients with the specified doses which is time-consuming and costly. With the aim of improving safety and acceptability to isoleucine supplementation in paediatric MSUD patients (aged 3 – 16 years), the research team decided to use 3D printing for preparing personalized therapies.
“There is a real need for new manufacturing systems to prepare personalised medicines. The potential application of the 3D printing for rare diseases are countless and it could help to make the medicines safer and more attractive to children,” stated Maria-Luz Couce, Head of the Paediatrics Section of the Clinical University Hospital concluded.
Personalised Printlets
FabRx’s 3D printing system can make Printlets with up to 6 different drugs, where each drug is represented as a different color. Compared to forming capsules by manual compounding, 3D printing the chewable formulations is faster and allows the preparation of one month’s therapy (28 printlets) to be completed in less than 8 minutes.
After 6 months of monitoring MSUD patients, the results showed that the personalized Printlets were as effective as the conventional medication in controlling the patients’ blood levels of isoleucine. Furthermore, isoleucine blood concentrations were closer to the isoleucine target value with less variation for the patients receiving the Printlets treatment. The ability of the 3D printing system to create Printlets with different colors and flavors had a further positive impact in enhancing patients’ acceptability of the treatment.
“Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients” is published in the International Journal of Pharmaceutics. It is co-authored by Alvaro Goyanes, Christine M.Madla, Aysha Umerji, Goretti Duran Piñeiro, Jose Maria, Giraldez Montero, María Jesús, Lamas Diaz, Miguel Gonzalez Barcia, Farhan Taherali, Paula Sánchez-Pintos, Maria-Luz Couce, Simon Gaisford, and Abdul W.Basit.
For more 3D printing news updates subscribe to our 3D Printing Industry Newsletter, follow us on Twitter and like us on Facebook. Visit 3D Printing Jobs for new opportunities in your area.
Featured image shows chewable Printlets (3D printed tablets) in different flavors, color, and doses. Photo via FabRx.



