Nominations for the 2021 3D Printing Industry Awards are now open, have your say who is leading the industry now.
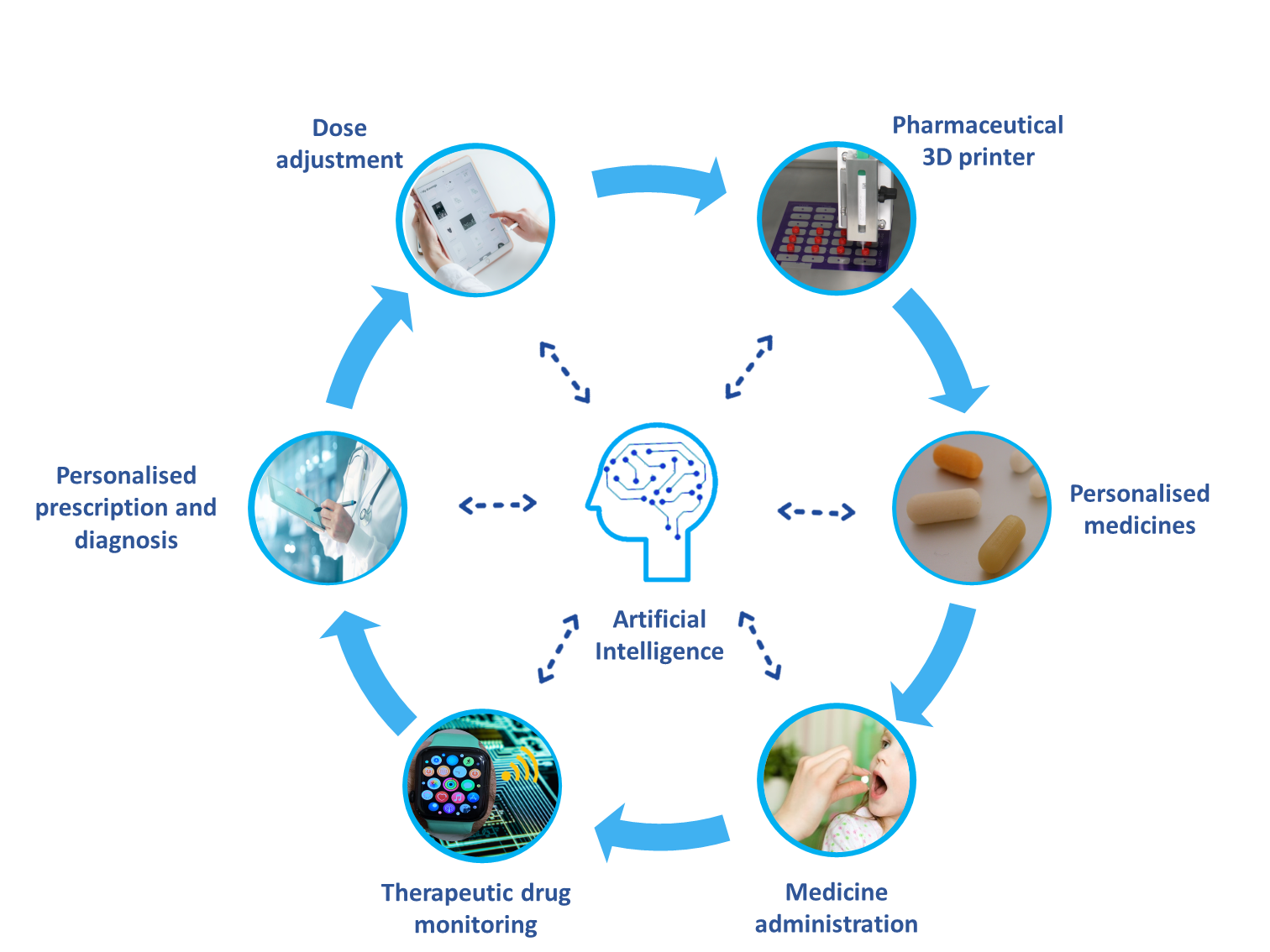
“3D printing will be a basic tool in the virtuous cycle of personalized medicine.”
That’s the view of Alvaro Goyanes, Co-Founder and Director of pharmaceutical 3D printing specialist FabRx, whose recent co-authored paper evaluates both the state-of-play of 3D printed pharmaceuticals and the clinical potential of the technology.
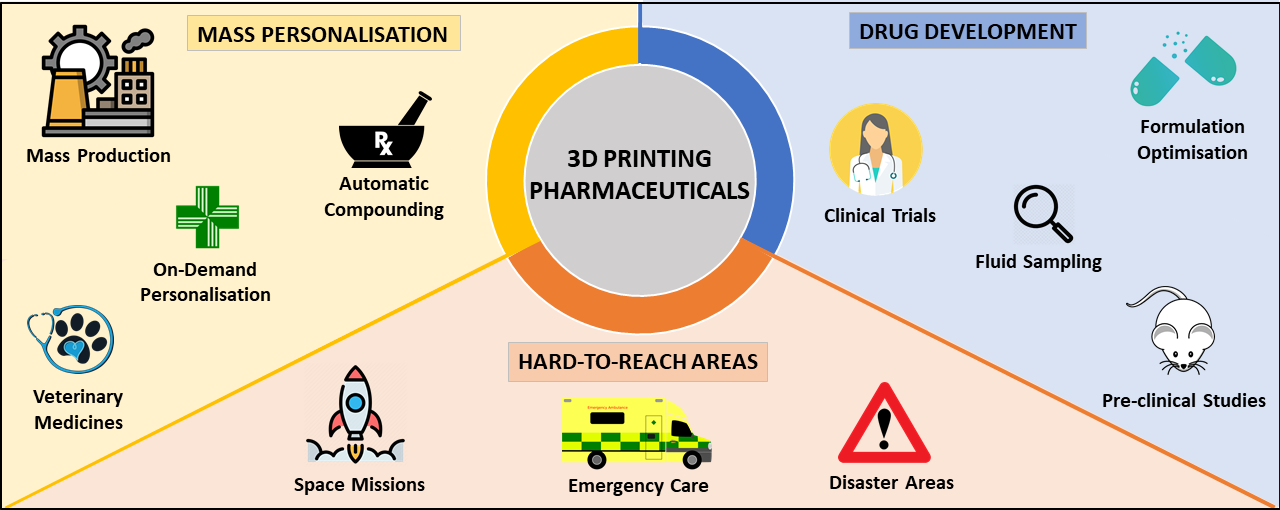
The paper states that 3D printing offers several significant advantages for clinical pharmaceutical drug development, not least the ability to personalize medicines to individual patient needs, expedite drug delivery timelines, and provide on-demand medication to those in hospitals, pharmacies and hard-to-reach areas.
However, while these benefits have been widely demonstrated in recent years, the clinical potential of the technology is yet to be realized.
“Despite this growing support for printing technologies, regulatory and technical challenges still remain before the widespread adoption of this technology into the pharmaceutical industry will occur,” the paper states.
3D Printing Industry spoke to paper co-author, Goyanes and Anna Worsley from FabRx about the findings of the study and garnered insights from Christoph Huels, Founder Additive Manufacturing of Tablets at the Innovation Center of global pharmaceutical company Merck, and Head of Excipient Solid Application at Merck’s Life Science Business Sector, Finn Bauer, on how 3D printing pharmaceuticals can transition from the lab to real-world clinical applications in the coming years.
Additionally, Kirk Donaldson, Vice President Business Development & Alliance Management at Aprecia, the company behind the first FDA-approved 3D printed drug Spritam, shares why the company believes “it’s important for people to begin seeing 3D printed pharmaceuticals as modern technology” that is available today, as opposed to future technology.
3D printing pharmaceuticals
3D printing can revolutionize the production of pharmaceuticals that target the gastrointestinal tract by offering a flexible drug manufacturing platform that can be adapted in response to changing markets and patient needs. Additive manufacturing makes possible the on-demand printing of personalized medicines on the front line in surgeries, hospitals, and hard-to-reach areas, while addressing issues of dosage inflexibility, high time and labor requirements, and large costs associated with conventional large-scale manufacturing processes, like tableting and encapsulation.
“Pharmaceutical 3D printing has many applications, we can say that it brings the manufacturing process closer to the patient, benefiting many different patient groups,” explains Goyanes. “It allows for the personalized dosing of drugs, removing the need to self-prepare medication or take multiple pills to each the prescribed, non-commercialized dose, an issue that arises for many treatment pathways that can lead to adverse effects. Pharmaceutical 3D printing also allows for personalized polypills, pills with multiple drugs combinations. This would benefit complex disorders and comorbidities.”
However, despite the widely demonstrated benefits of 3D printing pharmaceuticals, the clinical potential of the technology is yet to be realized, according to FabRx’s recent study. The paper highlights the main 3D printing technologies that are currently being leveraged for producing pharmaceuticals – binder jetting, vat polymerization, powder bed fusion (PBF), material jetting, and material extrusion.
“Each of the 3D printing technologies have different pros and cons, each being better suited to different specific applications, drugs and patient groups,” continues Goyanes. “Extrusion-based technologies like fused deposition modeling (FDM), semisolid extrusion or direct powder extrusion are probably more suited for printing at the dispensing point in hospitals and pharmacies. Other technologies included in vat photopolymerization like digital light processing (DLP) and stereolithography (SLA), could be more suitable for printing drug-loaded medical devices.”
Binder jetting, for instance, forms the basis of Aprecia’s ZipDose additive manufacturing technology that produces its Spritam tablets. Binder jetting is particularly suited to producing highly porous, fast-dissolving tablets with high drug-loading profiles such as Spritam, but can also be applied for the production of complex formulations such as near zero-order release dosage forms. Vat polymerization technologies such as SLA, DLP and continuous liquid interface production (CLIP) are often chosen for the fabrication of drug delivery devices with complex structures, such as microneedles for skin delivery, bladder devices for intravesical drug delivery, hearing aids, and drug-loaded scaffolds, among other applications.
Elsewhere, PBF has been mostly restricted to the production of medical devices such as scaffolds due to the risk of drug degradation from the high localized temperatures required to sinter powder materials. However, last year FabRx successfully used an SLS 3D printing technique to fabricate orodispersible tablets designed with Braille and Moon patterns to aid medicine taking for patients with visual impairment.

Material jetting techniques have been deployed for the production of oral films, nano and microparticles with arbitrary geometries, and controlled-release tablets, while material extrusion technologies such as FDM have been used to manufacture hollow structures and tablets with different shapes. FDM also enables the modification of drug release patterns to vary the infill percentage and the controlled and immediate release dosage forms of 3D printed tablets.
“In the future it would be ideal to have several technologies available for clinical applications, preferably combined into one printer,” Goyanes adds. “This would allow for fully personalizable medications for more treatment pathways.”
FabRx has already developed what it claims is the first GMP (good manufacturing practice) ready pharmaceutical 3D printer, the M3DIMAKER, which is currently available for research purposes. Before the machine’s launch in 2020, the firm had already completed trials with the printer in hospitals, pharmacies, and research institutions around the globe.
“The M3DIMAKER is already taking part in national and international collaborations which will involve future clinical trials, and we are in communication with the MHRA to aid regulation development for this state-of-the-art technology,” Goyanes says. “It combines multiple 3D printing technologies into one machine, allowing for flexible use and increased personalization potential.”

3D printed pharmaceuticals in industry
In addition to the surge in research interest surrounding 3D printed pharmaceuticals in recent years, there have been a number of notable developments from companies looking to commercialize the technology, too. One such firm is Merck, which in 2020 embarked upon a joint project with EOS Group company AMCM to develop and produce 3D printed tablets, first for clinical trials and then later for commercial manufacturing.
“There are several initiatives ongoing related to 3D printing of oral solid dosage forms,” says Bauer. “Different 3D printing technologies, such as powder jetting, material extrusion, and laser sintering are explored at Merck. In addition, different excipients are tested and developed for their use in extrusion-based applications. Parteck MXP is a polymer for hot-melt extrusion in our Life Science Sector and its use in 3D printing has been described in various publications.”
Founded way back in 1668, Merck is a significant Big Pharma player. The company’s growing interest in leveraging 3D printing for medicine development is therefore notable and could help to pave the way for greater commercialization of the technology in the future.
“Laser sintering is explored for the production of oral dosage forms in the Merck Innovation Center,” adds Huels. “We aim to make services available for the formulation of manufacturing of clinical trial supply in a first step. The team is planning to offer the technology to customers for exploratory studies in the course of the next year. In parallel, developments are on the way to build a 3D printer, able to work in a GMP environment. The GMP solution is expected to be ready for market introduction in two-to-three years.”
However, Huels points out that certain challenges still remain to scale up the 3D printing of medicines for commercial manufacturing, and fully translate the technology from the lab to clinics on the ground.
“Commercial manufacturing requires for so-called ‘blockbuster drugs’ and billions of tablets per year for global supply,” he says. “3D printing technologies are not able to secure the supply with the current throughput today. Significant improvements need to be done to reach that in the longer-term future. Nevertheless, the current throughput is already, or will be soon, well suited to supply orphan or smaller oncology indications, where only millions of tablets are required per year.”
In fact, Huels observes clinical supply as an application that can already be met by some 3D printing technologies, since only thousands of tablets are needed per clinical trial phase.
“Several developments are on the way to apply 3D printing for the drug development process,” he continues. “Therefore, it will only take a few years until more than the current drug (Spritam) will be supplied to patients via 3D printing. Smaller indications will be first, in which the use of 3D printing will have the most commercial viability.
“With further improvements in throughput and the decentralization of pharma production, a more generalized use of 3D printing for production may be observed.”

Achieving commercialization of 3D printed drugs
At the head of the field, the commercial potential of 3D printed pharmaceuticals is already being realized by Aprecia, with the company having received FDA approval for its 3D printed epilepsy medication Spritam back in 2015. The company has continued to innovate in this area since then, and in January entered into a partnership with non-profit R&D firm Battelle to increase its manufacturing output and advance its 3D printing system from clinical supply to commercial scale.
“Aprecia is actively engaged in exciting 3D printing research and development in several key areas including 3D printing formulation platforms for advanced, patient-centric dosage forms and the engineering of proprietary 3D printing systems based on improved processes and novel equipment trains,” says Donaldson. “Our first formulation platform (ZipDose) and enabling open-bed printing system is not only clinic ready, but also commercial ready.”
The company’s second formulation platform, ZipCup, reportedly expands Aprecia’s ability to handle a diverse set of materials while minimizing formulation time and risk. The platform, which Donaldson says will be clinic-ready within the next year and commercial-ready shortly afterward, can be delivered through either the original open bed or new in-cavity printing systems with some added process steps and equipment modifications. Regarding products, Donaldson says Aprecia has “several pipeline candidates” that will advance to clinic this year and next.
Since receiving FDA approval for Spritam, the company has closely observed how the 3D printed drug has been received by medical professionals, caregivers and patients.
“Neurologists have accepted Spritam as an alternative dosage form of levetiracetam for epilepsy patients that have difficulty swallowing tablets or do not like to take the liquid form,” Donaldson says. “Patients and their caregivers appreciate the rapid disintegration with a small sip of liquid and seem to remain both satisfied and persistent on the medication due to its ease of use. This has been the case across all age ranges.”
Regarding children, Donaldson says Spritam is an excellent way to bridge the gap from messy, difficult to measure, and sometimes unpleasant tasting liquid that they tend to start on, to a solid oral form as they get older.
“When introducing alternative dosage forms into a largely genericized market, both commercial and government payers may seek limits or restrictions to use so it’s imperative to make sure the overall value proposition resonates, and both physician and patient is supported and assisted with respect to coverage and reimbursement throughout the process,” he adds.
According to Donaldson, the real value of 3D printing is its ability to do things beyond the capabilities of conventional manufacturing technologies, such as overcoming formulation challenges and delivering personalized medicine closer to the site of care. However, he believes that further awareness of the technology’s potential for such applications still needs to be generated.
“There is always a need for greater awareness of the benefits to be gained with 3D printing technologies,” Donaldson says. “We need to expand awareness across the pharmaceutical industry at all levels of the organization through education and promotion. We need to generate more trial usage through feasibility studies to demonstrate our readiness. We need to gain market acceptance of novel dosage forms and personalized formulations through preference studies, market research projects, key opinion leader development, collaborations with other enabling pharmaceutical technologies, and ultimately successful in market products.”

Making the move from lab to clinic?
According to FabRx’s study, the integration of 3D printing alongside other novel technologies into pharmaceuticals is forecast to give rise to a “new digital pharmacy era”. The technology has the potential to provide a digital and decentralized platform for the production of tailored medicines that could be delivered at the point of care. However, while new research papers evidencing the potential of 3D printing technologies in this area are being published almost daily, only a comparatively small number of studies have been performed in pre-clinical and clinical settings.
Regulatory, quality, and technical concerns, as well as some resistance to digital technologies in the Pharma sector, are likely to be the reasons for this, as to date Spritam remains the only 3D printed drug product approved for commercialization by the FDA. From a Big Pharma perspective, one concern surrounding digitalized 3D printing technologies for decentralized production may be the protection of formulation and process details, as well as issues surrounding data security and accessibility.
With well-established manufacturing processes developed more than two centuries ago, the pharmaceutical industry is also facing a mindset and culture shift in order to meet the growing demand for personalized oral medicines. The study suggests that while there is still some work to be done before all stakeholders have confidence in 3D printing for drug development, inviting major stakeholders, including clinicians, patients and Big Pharma, to come together in a multidisciplinary approach to increasing the adoption of 3D printing in the pharmaceutical sector could further pave the way forward for the technology into human and animal studies.
“In the next 10 years, we predict that pharmaceutical 3D printing for personalized dosing and drug combinations will become standard practice for a handful of more troublesome treatment pathways around the world,” Goyanes concludes. “As more and more medication and drug combinations are tested, pharmaceutical 3D printing will become more widespread and will enter local pharmacies for more common treatment pathways.
“3D printing will be a basic tool in the virtuous cycle of personalized medicine. It will simplify and personalize many treatments, improving lives and saving healthcare organizations millions of pounds every year.”
Further information on FabRx’s study into the state of play of pharmaceutical 3D printing can be found in the paper titled “Translating 3D printed pharmaceuticals: From hype to real-world clinical applications,” published in the Advanced Durg Delivery Reviews journal. The study is co-authored by I. Seoane-Viaño, S. Trenfield, A. Basit, and A. Goyanes.
Nominations for the 2021 3D Printing Industry Awards are now open, have your say who is leading the industry now.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows Aprecia 3D printed pills. Photo via Aprecia Pharmaceuticals.



