Researchers in Korea and Hong Kong have used a modified 3D cell printing technique to fabricate a biomimetic blood vessel that was successfully implanted in a living rat.
According to the researchers, their approach to tissue-engineered biomimetic blood vessels outlined in their study provides a promising route for the construction of durable small-diameter vascular grafts. These 3D cell printed vascular grafts, which are used to redirect blood flow from one area of the body to another, have the potential to be used in future treatments of cardiovascular diseases.
“The artificial blood vessel is an essential tool to save patients suffering from cardiovascular disease,” comments Ge Gao, an author on the paper. “There are products in clinical use made from polymers, but they don’t have living cells and vascular functions.”
“We wanted to tissue-engineer a living, functional blood vessel graft.”
Creating functional vascular grafts using triple-coaxial cell printing
The researchers set out by explaining that cardiovascular disease is one of the leading causes of mortality worldwide, and requires over one million vascular bypass/replacement surgeries annually in the United States alone.
Tissue engineering, according to the authors of the paper, has emerged as a promising approach for creating viable small-diameter vascular grafts, which can be used to treat cardiovascular diseases through vessel implantation. However, thus far the construction of these vascular substitutes with both endothelial and muscular tissues has not been demonstrated.
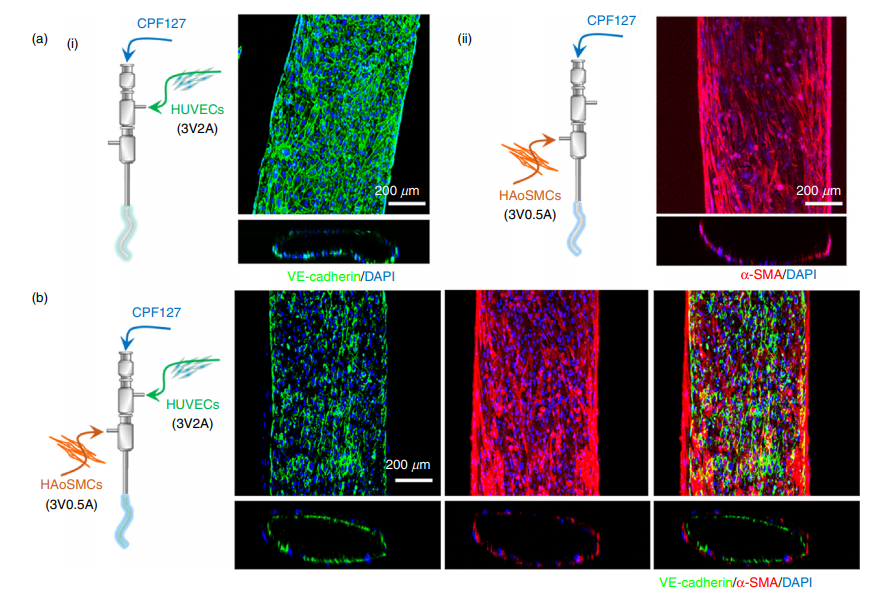
The localization of endothelial cells and smooth muscle tissues is essential for the grafts to function. As explained by the authors of the paper, they state: “To produce grafts with regular vascular functions, reconstruction of two critical constituents must be achieved, namely: (1) a confluent and quiescent endothelium offering a nonthrombogenic interface to inhibit thrombosis, and (2) contractile smooth muscle tissues that can withstand hemodynamic stress, exhibit physiological compliance, and adapt to local blood pressure changes via constriction and relaxation.”
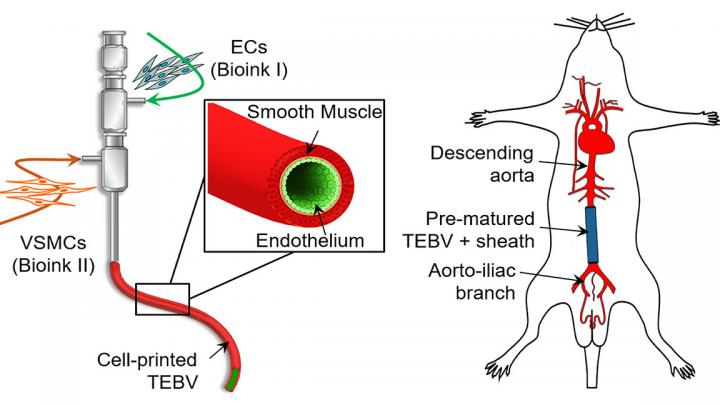
To overcome this hurdle, the researchers identified 3D cell printing due to its ability to construct tissue/organ equivalents. The coaxial-extrusion technique in particular, which uses a special nozzle to feed one type of material into another as it is extruded, enables the creation of vessel-like structures.
In order to successfully cell-print a functional tissue-engineered blood vessel, it was crucial for the research team to select cell-favorable bioinks that could both promote the cell functionality and enable the direct fabrication of vessels. Therefore, they developed a vascular-tissue-specific bioink formulated from smooth muscle cells from a human aorta and endothelial cells from an umbilical vein.
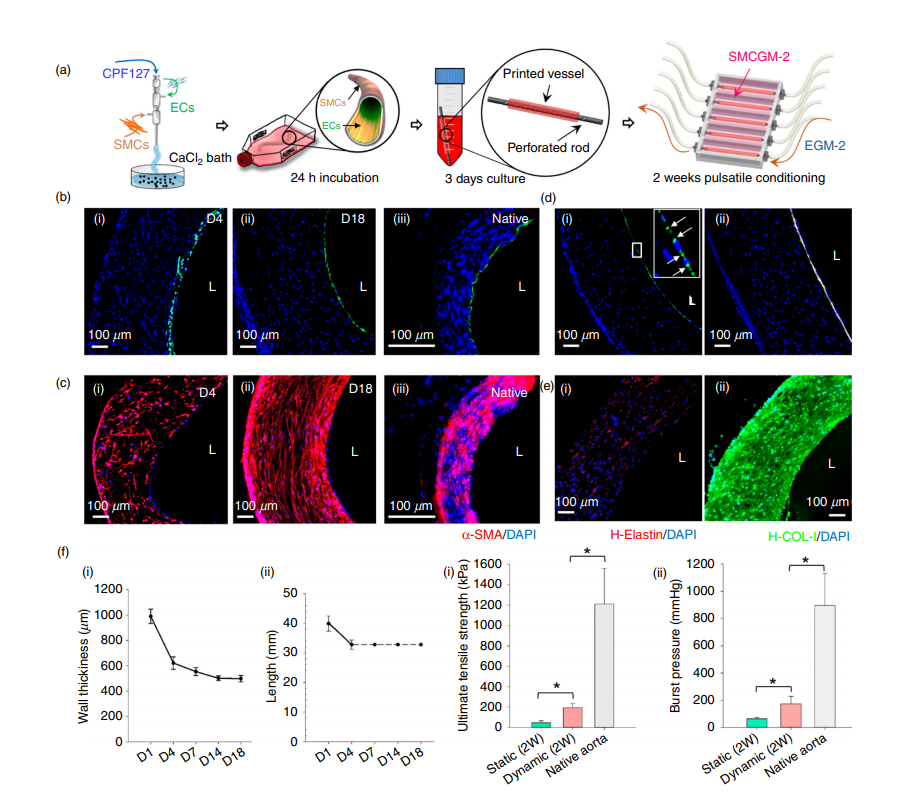
Combining this bioink formulation with a triple-coaxial cell printing (RTCCP) technique thus enabled the researchers to create a functional blood vessel with a dual-layer architecture that outperforms existing engineered tissue: “The developed triple-coaxial-cell-printing technique enabled the direct construction of vascular substitutes that contain both the endothelial and muscular layer,” explain the researchers.
These blood vessels were grafted as abdominal aortas into six rats and monitored over several weeks. The scientists observed a transformation in which the rat’s fibroblasts formed a layer of connective tissue on the surface of the implant, allowing the fabricated vessel graft to integrate as part of the existing, living tissue. Concluding the paper, the authors stated: “These findings revealed that the vascular equivalent constructed using the presented technique is a promising choice for tissue-engineering small-diameter blood vessel grafts.”
Moving forward, the researchers plan to continue developing their process leveraging the RTCCP technique alongside the vascular tissue-specific bioinks, specifically to increase the strength of the blood vessels closer to that of human coronary arteries. They also aim to perform long-term evaluation of vascular grafts, in order to determine what happens as they continue to develop in place and become real tissue in the implanted environment.
3D bioprinting in tissue engineering research
Tissue engineering is a rapidly evolving field, and the latest advances in 3D printing and bioprinting has enabled the technology’s application in research projects seeking to build living tissue constructs in the shape of human organs.
For example, in late 2019, researchers from Harvard University’s Wyss Institute developed a novel sacrificial ink-writing technique called SWIFT (sacrificial writing into functional tissue) to 3D print large, vascularized human organ building blocks (OBBs). The researchers demonstrated the method by creating cardiac tissue that fuses and beats synchronously over a 7-day period.
Around the same time, researchers at Lehigh University in Pennsylvania also presented a new 3D printing platform that facilitated the regeneration of multiple tissues using spatially functionalized scaffolds. The study details how 3D printing highly organized biomaterial scaffolds can be used to regenerate two different tissues.
Just recently, in February 2020, we reported on a study from New Jersey-based Rutgers University where engineers had developed a new adaptable bio-ink made of living cells that could be used to 3D print scaffolds for human tissue growth.
The study discussed in this article, “Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing,” is authored by Ge Gao, Hyeok Kim, Byoung Soo Kim, Jeong Sik Kong, Jae Yeon Lee, Bong Woo Park, Su Hun Chae, Jisoo Kim, Kiwon Ban, Jinah Jang, Hun-Jun Park and Dong-Woo Cho. The research was supported by grants funded by the Korean government, the Ministry of Trade, Industry & Energy (MOTIE, Korea), and the Ministry of Science, ICT and Future Planning. It is published in Applied Physics Reviews.
The nominations for the 2020 3D Printing Industry Awards are now open. Who do you think should make the shortlists for this year’s show? Have your say now.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows researchers in Asia used triple-coaxial cell printing technology to construct biomimetic tissue-engineered blood vessels that were evaluated in vivo through an interpositional abdominal aorta graft in a rat model. Image via Applied Physics Reviews.

