Researchers from Harvard University’s Wyss Institute have developed a novel sacrificial ink-writing technique called SWIFT (sacrificial writing into functional tissue) to 3D print large, vascularized human organ building blocks (OBBs).
Demonstrating its method, the team has created cardiac tissue that fuses and beats synchronously over a 7-day period. This enables the rapid assembly of perfusable patient and organ-specific tissues at therapeutic scales.
“Our SWIFT biomanufacturing method is highly effective at creating organ-specific tissues at scale from OBBs ranging from aggregates of primary cells to stem-cell-derived organoids,” said Jennifer Lewis, corresponding author at the Wyss Institute.
“By integrating recent advances from stem-cell researchers with the bioprinting methods developed by my lab, we believe SWIFT will greatly advance the field of organ engineering around the world.”
Reducing transplant wait times with additive manufacturing
According to the researchers, in the U.S., approximately 20 people die every day waiting for an organ transplant. While more than 30,000 transplants are now performed annually, there are reportedly over 113,000 patients currently on organ waitlists. To resolve this organ shortage, scientists are betting their hopes on artificially grown human organs.
Tissue engineering is a rapidly evolving field. Advances in 3D printing have led to a boom in using that technique to build living tissue constructs in the shape of human organs. Organ building blocks composed of patient-specific-induced pluripotent stem cell-derived organoids offer a pathway to achieving tissues with the requisite cellular density, microarchitecture, and function. However, to date, little attention has been devoted to their assembly into 3D tissue constructs.
By 3D printing vascular channels into living matrices composed of stem-cell-derived OBBs, the team’s SWIFT technique overcomes this major hurdle and yields viable, organ-specific tissues with high cell density and function. “This is an entirely new paradigm for tissue fabrication,” said co-first author Mark Skylar-Scott, Ph.D., a Research Associate at the Wyss Institute.
“Rather than trying to 3D print an entire organ’s worth of cells, SWIFT focuses only on printing the vessels necessary to support a living tissue construct that contains large quantities of OBBs, which may ultimately be used therapeutically to repair and replace human organs with lab-grown versions containing patients’ own cells.”

SWIFT biomanufacturing
SWIFT is a two-step biomanufacturing process that begins with assembling hundreds of thousands of these OBBs into living matrices with high cellular density into a dense, living matrix of OBBs. Contains about 200 million cells per milliliter, the OBB matrices used for SWIFT also have to exhibit the desired self-healing, viscoplastic behavior.
In the second step, perfusable vascular channels are embedded within the matrix by writing and removing a sacrificial ink (i.e. embedded 3D bioprinting). The vascular network constructed allow oxygen and other nutrients to pass through, delivering these vital substances to cells.
“Forming a dense matrix from these OBBs kills two birds with one stone: not only does it achieve a high cellular density akin to that of human organs, but the matrix’s viscosity also enables printing of a pervasive network of perfusable channels within it to mimic the blood vessels that support human organs,” added co-first author Sébastien Uzel, Ph.D., a Research Associate at the Wyss Institute and SEAS.
How to make a beating heart
The cellular aggregates used in the SWIFT method are derived from adult induced pluripotent stem cells. Mixed with a tailored extracellular matrix (ECM) solution, the aggregate makes a living matrix that is compacted via centrifugation.
At cold temperatures (0-4°C), the dense matrix has the consistency of mayonnaise. Soft enough to manipulate without damaging the cells, the matrix is still thick enough to hold its shape – the perfect medium for sacrificial 3D printing. In this technique, a thin nozzle moves through this matrix depositing a strand of gelatin “ink” that pushes cells out of the way without damaging them.
Heated to 37 °C, the cold matrix gradually stiffens to become more solid. As temperature increases, the gelatin ink melts and can be washed out. This leaves behind a network of channels embedded within the tissue construct that can be perfused with oxygenated media to nourish the cells. The researchers were able to vary the diameter of the channels from 400 micrometers to 1 millimeter. The 3D printed channel can be seamlessly connected to form a branching vascular network within the tissues as well.
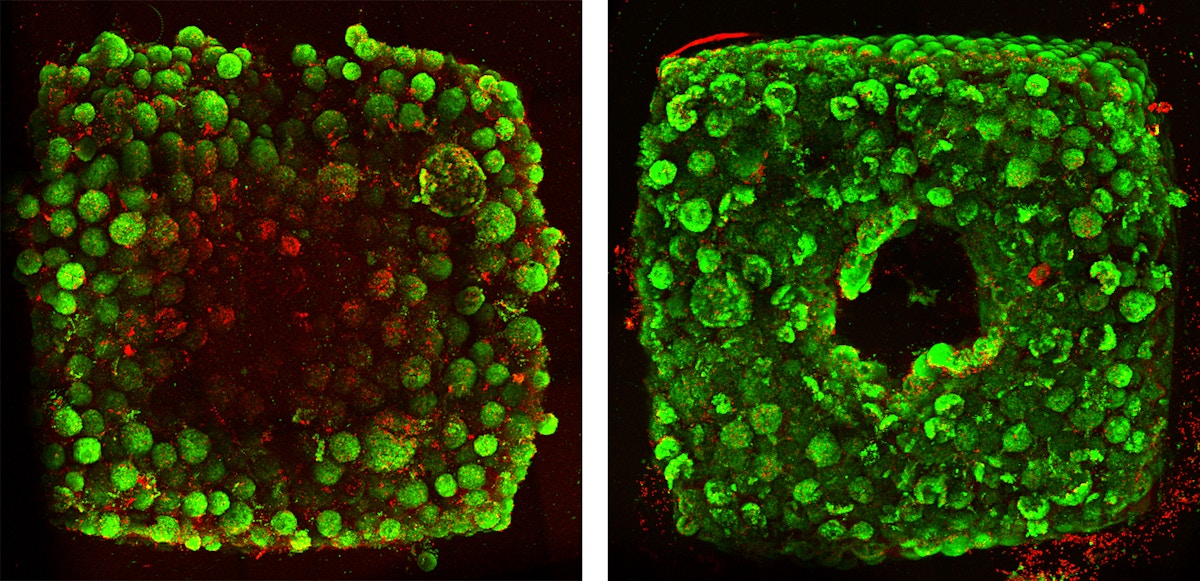
The SWIFT future in therapeutic applications
To determine whether the tissues displayed organ-specific functions, the team 3D printed, evacuated, and perfused a branching channel architecture into a matrix consisting of heart-derived cells. After fabricating the heart-like structure, media was flown through the channels for over a week. During that time, the cardiac OBBs fused together to form a more solid cardiac tissue. The contractions became more synchronous and over 20 times stronger, mimicking key features of a human heart.
In the future, the team envisions adopting new protocols that offer a pathway to create more mature, micro vascularized OBBs. Collaborations are underway with Wyss Institute faculty members Dr. Chris Chen at Boston University and Dr. Sangeeta Bhatia at MIT.
“Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels” is published in Science Advances. It is co-authored by Mark A. Skylar-Scott, Sebastien G. M. Uzel, Lucy L. Nam, John H. Ahrens, Ryan L. Truby, Sarita Damaraju, and Jennifer A. Lewis.

Subscribe to our 3D Printing Industry Newsletter and follow us Facebook and Twitter for the latest additive manufacturing updates.
Visit our 3D Printing Jobs board to find out more about opportunities in additive manufacturing.
Featured image shows living embryoid bodies surround a hollow vascular channel printed using the SWIFT method. Photo via Wyss Institute at Harvard University.



