Professor Jennifer Lewis and her group in the School of Engineering and Applied Sciences at Harvard University, are behind some of the biggest stories in the 3D printing industry. In 2016 alone Lewis et al., in collaboration with Wyss Institute for Biologically Inspired Engineering, released papers demonstrating breakthrough developments in 3D printing living kidney and heart cells that are poised to revolutionise the medical industry.
The Lewis Labs’ 3D printed beating heart-on-a-chip. Clip via Harvard University.
Additionally, the team’s research has developed methods for 3D printing in thin-air, and constructing microbatteries to power wireless devices.
In this interview with Professor Lewis, 3D Printing Industry hear how knowledge from each of these fields converge as one, interminable, driving force to advance 3D printing and material possibilities.
Starting with ceramics
Breakthroughs from the Lewis Lab are built upon 2 decades of work in the field that started in ceramics, as Professor Lewis explains;
Our first work in the area of tissue engineering was work that we did with collaborators at NYU many years ago, I think the papers came out in the early 2000s […] And there we were printing bone scaffolds.
3D printed scaffolds are shapes that mimic the natural structure of bones or tissue in the body. When laden with biomaterial, the scaffolds help cells to perform a “sorting out” to grow in a similar way to how they would in the body. Professor Lewis adds,
We were using our techniques even back then to produce scaffolds that could go into a defect site in a bone if there’s injury, and then induce natural bone ingrowth and provide wound healing.
The material Lewis and her team used for the bone scaffolds in the early 2000s is hydroxyapatite – the calcium based mineral that makes up natural bone. She continues,
So that was our very first work, and of course hydroxyapatite is a ceramic material and it leveraged all of that capability. And over time we moved on from ceramic materials to polymers and hydrogels.
The shift to bioprinting
From working in polymers and hydrogels, Lewis then moved the lab from Illinois to a new opportunity at Harvard, and that’s where the biomedical aspect started to flourish. She says,
…it’s one thing to print acellular scaffolds, such as ceramics, synthetic polymers and hydrogels, it’s yet another to have the facilities and capabilities and knowledge to do cell culture, and to create cell-laden inks.
When I think of bioprinting, I really think that the bio part is key. It’s not just acellular, you need to be printing materials that contain living matter.
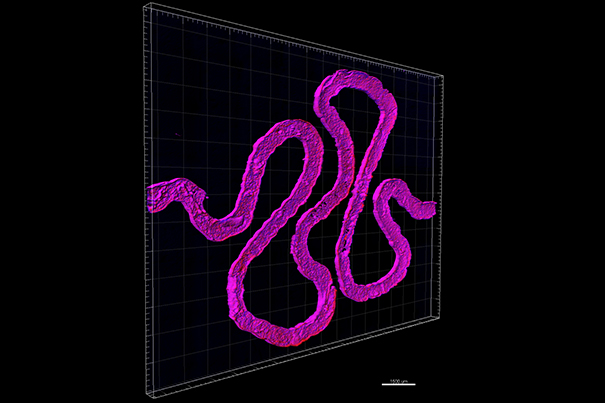
The future of organs-on-a-chip
In our conversation, Professor Lewis identifies two main challenges to working with living matter. First is what she calls the “front-end challenge” in simply making the inks, that doesn’t come with 3D printing hydrogels or polymers. In Lewis’ words this is,
…making sure that you have identified the appropriate cell types and that you’re maintaining them in cell culture, so that you can grow the cells that you need to create the cell-laden ink that can be printed.
Afterwards, when working with the inks, there is the additional pressure of time-sensitivity,
Within an hour or two hours of making these inks, the whole tissue needs to printed, and we need to be able to embed a vascular network so we can supply the cells with oxygen, with nutrients that they need to live.
Through using microfluidic channels the Lewis Lab has been able to create living organs on a chip that remain viable for up to one year at time, an unprecedented development that is leading them on to the next step.
The big challenge is going to be to allow those tissues to interact with the host […] The body has pretty strong immune system ad when it recognises something that shouldn’t be in the body, even if it’s made of similar materials it starts to attack the tissue, and undergoes a process called fibrosis where it starts basically walling it off from the rest of the body.
As we see a transition from bioprinting being used by pharmaceutical industry, for drug screenings, for mechanistic studies, to more studies where it’s going to be in vivo applications we’re really on the front end of that still.
For more exclusive interviews with 3D printing experts sign up to the 3D Printing Industry newsletter and follow us on social media.
Featured image shows Professor Jennifer Lewis in the lab Photo courtesy of Seth Kroll, Wyss Institute for Biologically Inspired Engineering at Harvard University



