Researchers at the Swiss Federal Institute of Technology Zurich (ETHZ) have produced a universal nanocarrier ink platform, that provides tailored rheology for extrusion‐based 3D printing, and facilitates the formulation of biofunctional inks.
The Universal Nanocarrier Ink (UNI), can be combined with a range of functional secondary polymers, to enable the stabilization of printed constructs via secondary cross‐linking. Applications for the material’s unique customized biofunctionality, include tissue engineering and drug delivery, as well as the rapid formulation of a broad range of functional inks, for the additive manufacturing of advanced biomaterials.
The need for a new type of ink
Efficient production using 3D printing often requires inks to be engineered in order to produce materials with specific properties, such as shear-thinning behavior, biofunctionality, or stimuli responsiveness. The inks also need to be able to satisfy the physical constraints of the machine they’re made for, such as material viscosity, flowability, self-healing rate, and gelation kinetics needed for the machine. If it is being used for tissue engineering, for example, the ink should be cytocompatible, so the target application of the substance could also introduce additional design parameters.
Direct ink writing (DIW), is a 3D printing technique that is commonly used for biofabrication, and operates by extruding an ink via a piston, pneumatic or screw-driven robotic dispensing in defined locations to fabricate a final 3D construct. In order to be compatible with this method of printing, inks need to display a shear-induced flow during extrusion, and rapid material reformation for shape retention following deposition. Stabilization of the structure following deposition is also needed for long-term use in vitro and in vivo, and this requires additional functionality for secondary cross-linking, to reinforce the formation.
While several efficient bioinks have been developed, the range of materials for DIW remains limited according to the researchers, and only a few current inks are versatile and tunable across multiple uses. This is likely due to the fact that new materials require significant design and formulation, but universal carrier inks offer the potential to be compatible with a broad range of post-curing materials. Such compatible materials can dramatically simplify the design and development of new customizable inks, and the researchers set out to innovate a UNI platform for DIW based on engineered polymer–nanoparticle assembly.
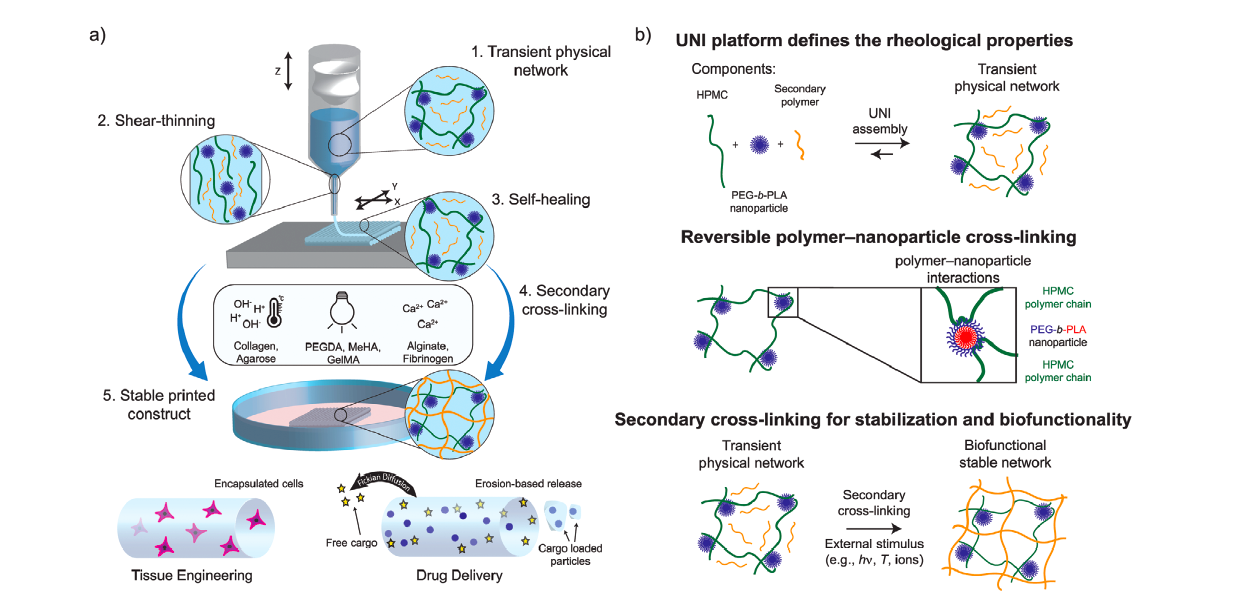
Creating the new bioink
The UNI was engineered using generally regarded as safe (GRAS) materials (PEG-b-PLA NPs and HPMC), and with rheology that was tailor-made for DIW. Stabilization was enabled by combining the base UNI with a range of functional secondary polymers. Merging the material with different polymers increased biofunctionality, and made the platform sufficiently robust to enable efficient DIW of all composite inks. The researchers had engineered a single universal nanocarrier ink that could be combined with a range of functional polymers for multiple biomedical applications, without additional chemical design or modification.
To demonstrate the potential and versatility of UNI-15 (a specific test variant of the material) to function as a universal carrier ink for DIW, composite inks were produced by mixing UNI-15 with various secondary polymers. While the UNI platform possessed excellent rheology for DIW, its long-term stability was poor owing to the transient and reversible polymer–nanoparticle cross-links. The researchers then mixed UNI-15 with functional polymers that could be crosslinked following deposition, to stabilize the printed construct post-fabrication. By combining a broad range of secondary polymers with the same nanocarrier ink, the printed constructs could be stabilized via secondary cross-linking photopolymerization, ionic gelation, or temperature-induced gelation.
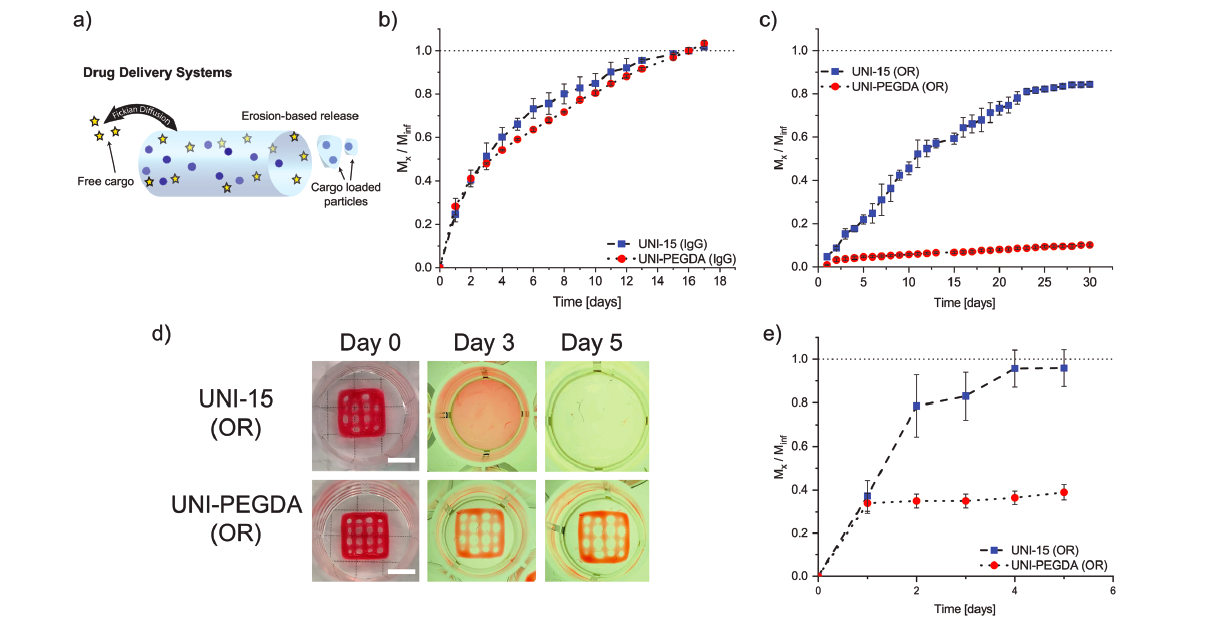
In order to demonstrate that the secondary polymers did not affect the rheological properties of the universal carrier ink, the same rheometric tests were performed on four of the composite materials containing UNI-15, and the corresponding secondary polymer. The addition of the secondary polymer did not inhibit the formation of a viscoelastic gel, for all inks tested and the transient physical networks were able to reform ≈75% in less than 60s. For tissue engineering and drug delivery applications, post-fabrication stability of printed constructs is necessary for shape retention and long-term functionality, and this was made possible by secondary cross-linking of the composite inks.
The researchers concluded that their UNI platform had successfully simplified ink engineering, and represents a facile and versatile method to develop new biomaterials and bioinks for 3D printing. The team did stress that all its components were required for the process to work though, because they each comprise viscous liquids, and do not provide suitable rheology for DIW, nor stability via secondary cross-linking. Nonetheless, using this approach, different ink formulations were designed specifically for tissue engineering and drug delivery applications. The newly-developed UNI demonstrated its ability to simplify the production of future 3D printing of complex biomaterials, in precision medicine.
Additive manufacturing and bioinks
Researchers from a number of institutions have attempted to perfect bioink technology, with the intention of utilizing the materials for medicinal applications.
Texas A&M University researchers announced that they had developed their own highly 3D printable bioink in February 2020. Known as Nanoengineered Ionic–Covalent Entanglement (NICE), the material was designed to overcome the structural stability deficiencies of current bioinks.
Biomedical engineers from Rutgers University in New Jersey developed a bioink in November 2019, which is designed to support growing human tissues. The researchers used modified versions of hyaluronic acid and polyethylene glycol to form a strong gel suitable for use as a scaffold, which could be adjusted depending on the mixture of ink used.
Researchers from Northwestern University and the Ann & Robert H. Lurie Children’s Hospital of Chicago developed a bioink for 3D printing bioprosthetic ovaries in December 2019. The ovaries were produced so that they could be implanted in infertile women, allowing them to bear children.
The researchers’ findings are detailed in their paper titled “Universal Nanocarrier Ink Platform for Biomaterials Additive Manufacturing,” published on November 25th, 2020 in the Nano Micro Small journal. The research was co-authored by Elia A. Guzzi, Giovanni Bovone, and Mark W. Tibbitt.
You can now nominate for the 2020 3D Printing Industry Awards. Cast your vote to help decide this year’s winners.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.



