Though Organovo produced 3D printed kidney cells earlier this year, last week, Professor Melissa Little‘s research team at the University of Queensland (UQ)’s Institute for Molecular Bioscience demonstrated the ability to create a complete miniature kidney. Now, however, the two are teaming up to create a mutually beneficial relationship, both for the partners involved and the world at large. Today, Organovo has signed an exclusive worldwide licensing agreement with UniQuest, the technology transfer and commercialization company of UQ, to patent applications related these kidney cells, developed from induced pluripotent stem cells (iPSCs).
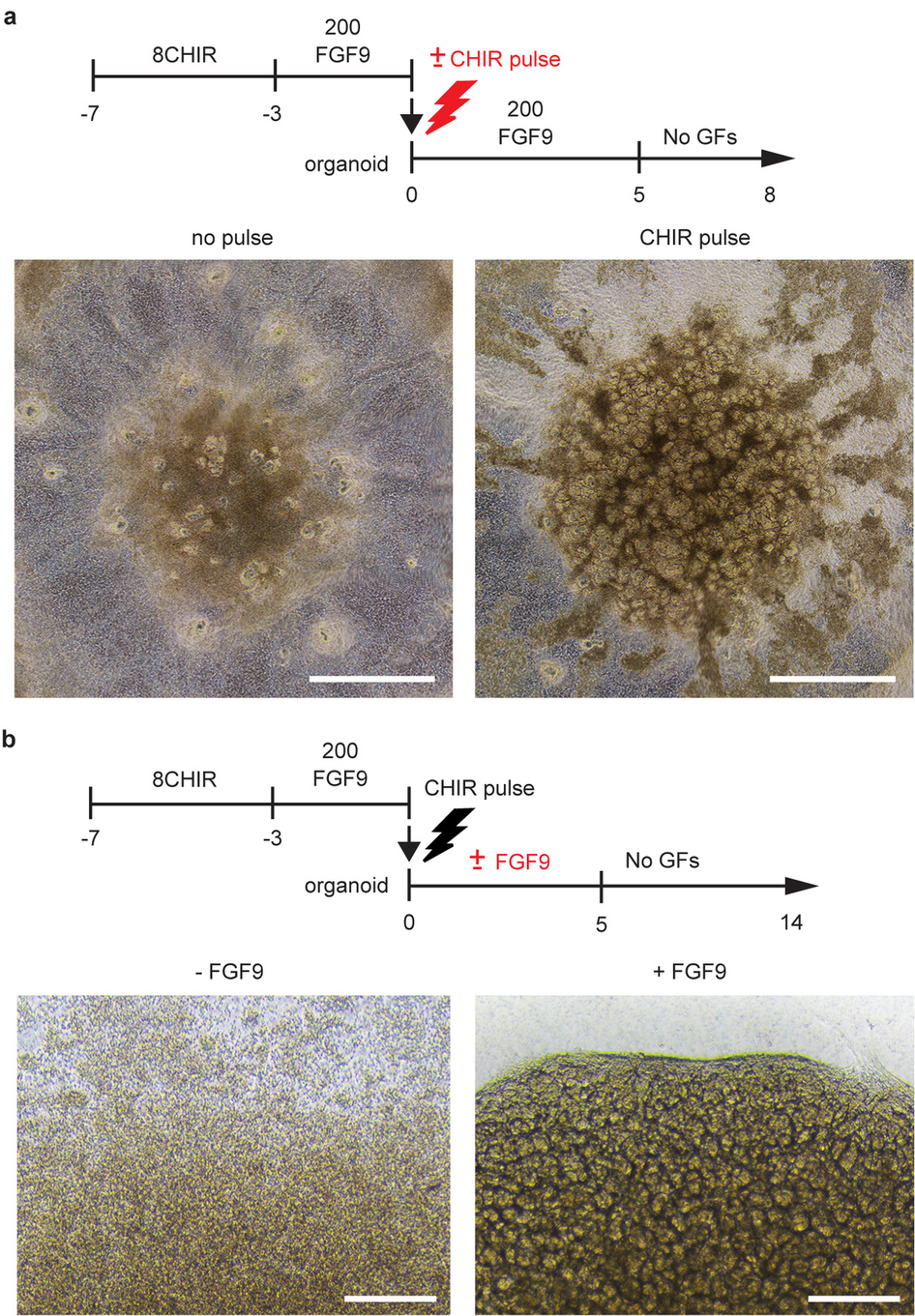
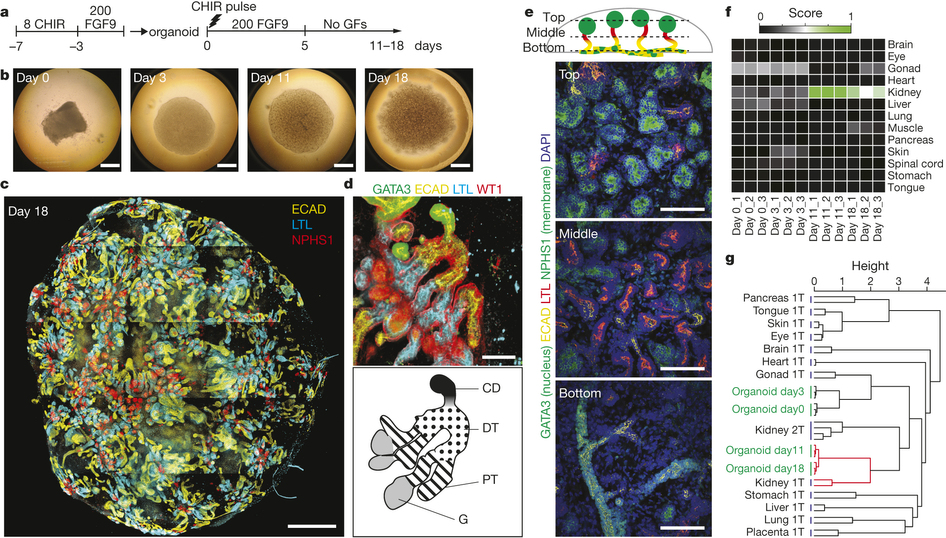
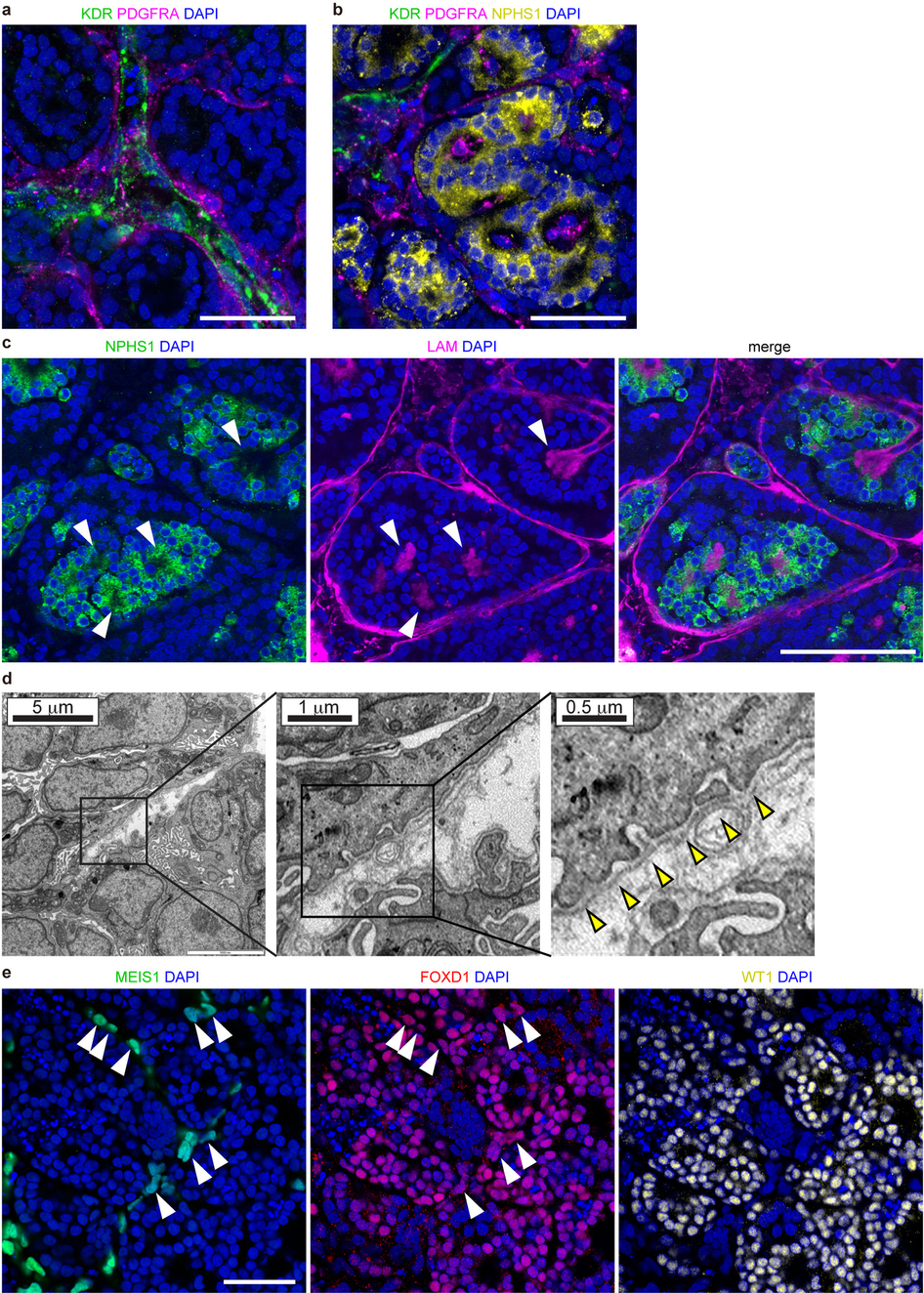
Building off of previous work to grow a “mini-kidney” containing two types of kidney cells, Prof. Little’s team published research outlining their groundbreaking ability to, relying on iPSCs, grow a mini-kidney made up of all of the cell types normally present in a human kidney, with the necessary vascularization, as well. Prof. Little says of the research, published last week in Nature, “The mini-kidney we have been able to grow is very complex and more like the real organ. This is important for drug testing as we hope these mini-kidneys will respond to the drugs as a normal organ might.”
It should, of course, be clarified that, though these lab-grown organoids contain all of the cells present in an actual kidney, they are only kidney models. The organoids measure only 1 cm across and are made up of only a fraction of the total number of cells in the organ itself. To create them, the team used signally factors to direct the iPSCs, derived from skin cells, to create two kidney progentior cell types. These cells then grew over the course of several weeks to create the organoids, consisting of nephrons, connective tissue, blood vessels, and collecting ducts. The resulting organoid is not dissimilar from the kidney of a developing human fetus.
Naturally (or Frankensteinifically?), Little’s research was a perfect fit for the only publicly traded bioprinting firm. Organovo will work with the team to develop IP for commercial applications for her research, such as kidney disease modelling, nephrotoxicity screening, and the research of new drugs to treat kidney disease. The professor’s research would also benefit the company’s existing 3D printed kidney proximal tubule tissue, which will be released as a commercial product next year. Relying on the technology licensed from UniQuest, the company would be able to release a complete kidney tissue product, significantly expanding on their own product. Organovo has been granted worldwide development and commercialization rights for in vitro applications of UQ’s technology, with UniQuest receiving an upfront technology access fee and the ability to receive milestone and royalty payments.
Chief Technology Officer at Organovo, Sharon Presnell, Ph.D., said of the deal, “We are excited to license this groundbreaking technology to enable the development of human kidney tissues that could change the landscape of drug testing and kidney research. Working with leading scientists such as Professor Little extends our leadership position in the generation and commercialization of tissues that better recreate in vivo human biology.”
UniQuest CEO Dr. Dean Moss commented, “This deal is anchored in world-leading induced pluripotent stem cell research by Professor Melissa Little and follows a research collaboration between The University of Queensland and Organovo, facilitated by UniQuest. We are delighted to work with Organovo so that they can further develop and commercialise the technology to accelerate the drug discovery process and enable treatments to be developed faster and at lower cost.”
This is obviously huge news for all parties involved, including the human race. While there are a number of bioprinting companies working towards bioficial organs, Organovo has previously focused mainly on the cells themselves for drug testing applications. UQ’s research, however, gives them a giant leap ahead and could, one day, enable the development of complete 3D printed organs. That day may still be some way off, but, when it comes, the 93,000 people on the kidney transplant waiting list will be able to receive transplants much sooner than the current waiting time of 5-10 years, thanks to the ability to fabricate patient-specific cells. In the mean time, those patient-specific cells can be used to discover new medicines to treat their diseases, a fact that has, today, increased Organovo’s stock price by 3.93%.



