Researchers from the University of Oxford have used 3D printing to improve upon the single-droplet resolution 3D bioprinting process, allowing them to create synthetic tissues with greater precision.
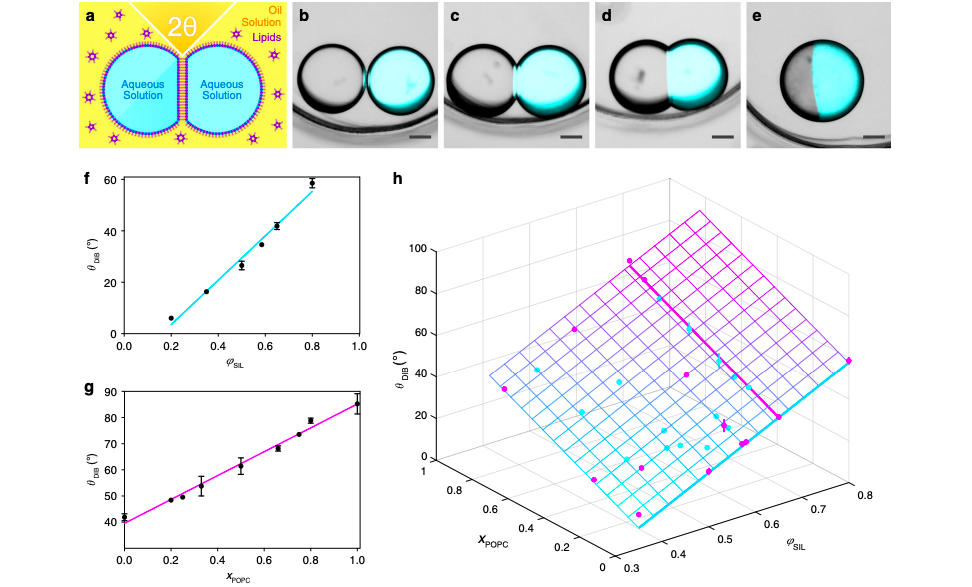
Optimizing the contact angle (θDIB) between a pair of droplets was found to be key in enabling the precise positioning of hundreds of picolitre-sized droplets within multi-layer networks. The technique could enable the fabrication of complex synthetic tissues where precisely positioned compartments perform coordinated tasks, such as adhesive giant unilamellar vesicles or protein compartments.
3D printing more efficient synthetic tissues
Using 3D printing to create droplet networks connected by interface bilayers, is an established method of producing synthetic tissues, but its functionality relies on precisely ordered structures. According to the researchers, the precision and consistency of these formations are currently limited, which restricts the intricate designs and complexity of functions that synthetic tissues are able to perform. In addition, networks of droplets have been produced using mechanical placement and microfluidics before, but the automation and scalability of 3D printing have made it the most efficient approach.
Using inspiration from space-filling polyhedra in nature such as honeycombs created by bees, the research team examined the properties and performance resulting from molecules packed into crystal structures. Their approach was summarily based upon the controlled close packing of deformable spheres, which they would use to create tissues that mimic the complex and cooperative structures and functions of living flesh. These networks of picolitre-sized droplets are desirable because they provide the advantages of discrete compartmentalization, inherent connectivity, and communication between subunits.
Droplet interface bilayers (DIBs) form the basis of the networks and are created when two aqueous droplets in oil are brought together and form a bilayer at the droplet–droplet interface. When these liquids are packed together in 3D, each makes multiple contacts (by forming DIBs with its neighbors), resulting in the deformation of the spherical droplets into polyhedra. In order to build such a 3D network, it is crucial to understand the parameters that direct packing and droplet deformation, and thereby affect printing resolution and fidelity.
The research team hypothesized that single-droplet-wide signaling pathways would be feasible if synthetic tissues could be patterned at single-droplet resolution. Identifying the composition of the liquids and angle of contact as defining factors in the process, the researchers found that in optimal θDIB, droplets could form hexagonal close-packed lattices.
The methodology behind single droplet resolution 3D printing
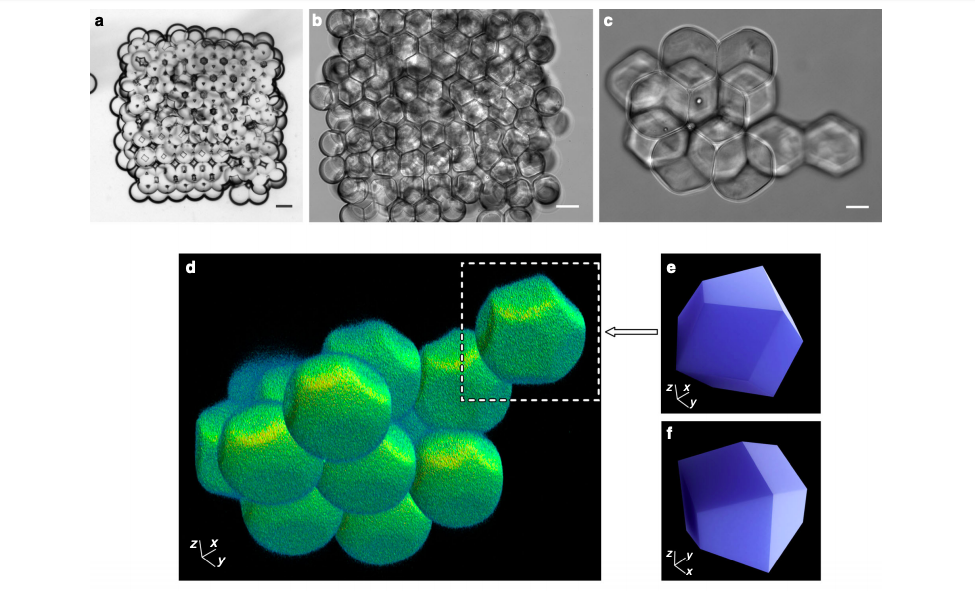
Using a custom-built 3D printer, the Oxford researchers constructed 3D droplet networks comprising hundreds of picolitre-sized aqueous droplets (PBS, 100 µm diameter, ≈524 pL volume). To form a network, 224 droplets were automatically generated at an ejection frequency of 0.5 s−1 and positioned line-by-line, layer-by-layer. After analyzing 129 printed networks, the research team established two prevalent regular packing arrangements that they classified as ‘hexagonal’ and ‘square,’ and were suitable for creating 3D lattices.
The Oxford research team found that 3D printed networks became hcp lattices when the DIB in droplet pairs approximated the geometrically derived critical angle (θc) of 35.3°. Deviations from that angle led to lattices that were either too loosely packed or tightly compressed and deformed, featuring an increased amount of defects. Regular packing of the first layer was also found to be critical to the lattice’s structural integrity because misalignment would lead to defects in the higher layers.
Improved control over the networks meant that the researchers were able to create more complex tubular designs and build synthetic flesh containing two single-droplet-wide conductive pathways. The tissue acted as proof of concept, but according to the research team, the process could be further improved by ‘annealing’ steps after the printing process, or by templating the droplet packing lattice using patterned surfaces. What’s more, the Oxford researchers believe that 3D printing’s automated advantages over other production methods make the technology readily scalable.
The technique could be applied in the precise construction of 3D printed structures containing eukaryotic and prokaryotic cells to study tissue development, cellular ecology, and disease models. Precise patterning and packing also provide the basis for accurately interfacing 3D-printed synthetic constructs with living tissues, offering new means to monitor, control, or complement biological function.
Additive manufacturing synthetic tissues
OxSyBio, a company spun out by the University of Oxford, has been developing similar bioprinting techniques since 2014, and managed to raise £10m in funding towards its research in March 2018. Having developed its own 3D bioprinter the year before, the business’ ambition was to create additive manufactured tissue for surgical applications.
Researchers from other institutions have also attempted to perfect the bioprinting process, including a team from the University of Illinois at Chicago (UIC) who developed a scaffold-free 3D bioprinting process in June 2019. The cell-only constructs could be printed in intricate forms and constructed using different cell types, without a hydrogel carrier or traditional scaffold to stabilize them.
In May 2018, a group of researchers from the University of California, Los Angeles (UCLA) developed their own 3D bioprinter that utilized a novel 3D printing technique. Using a microfluidic chip, the system was able to efficiently print multiple materials within a single process, and accelerate the deposition rate of material extrusion technology.
The researchers’ findings are detailed in their paper titled “Controlled packing and single-droplet resolution of 3D-printed functional synthetic tissues,” published on April 30th 2020 in the Nature Communications journal. The research was co-authored by Alessandro Alcinesio, Oliver J. Meacock, Rebecca G. Allan, Carina Monico, Vanessa Restrepo Schild, Idil Cazimoglu, Matthew T. Cornall, Ravinash Krishna Kumar and Hagan Bayley.
You can now nominate for the 2020 3D Printing Industry Awards. Cast your vote to help decide this year’s winners.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows graphs and diagrams that depict how changes in angle and composition of the droplets affect the integrity of the droplet network. Photo via Nature Communications.


