
Recently, a 3D bioprinting project conducted at the University of California, Los Angeles (UCLA) has been making headlines for its potential to create complex biological tissues.
Led by UCLA Professor of Engineering Ali Khademhosseini, the project describes a bioprinter that makes use of microfludic chip to efficiently print multiple materials in a single process.
Yu Shrike Zhang of Brigham and Women’s Hospital and Harvard Medical School, joins Khademhosseini on the project as co-senior author of the published results. In this exclusive interview, I speak to Zhang to learn more about this latest 3D bioprinting technique, and its potential to transform the future of medical research.
Multimaterial 3D bioprinting on a chip
Zhang is currently a Faculty and Associate Bioengineer within the Division of Engineering in Medicine at Harvard Medical School (HMS).
In this latest project, Zhang contributed to the work of a UCLA/HMS collaborative that sought to develop a new, more efficient technique of 3D printing biological matter. The product of this research is a stereolithography (SLA) based bioprinting platform.
In place of a typical build plate, the UCLA/HMS platform has a small, circular outlet in the center of a microfludic chip.
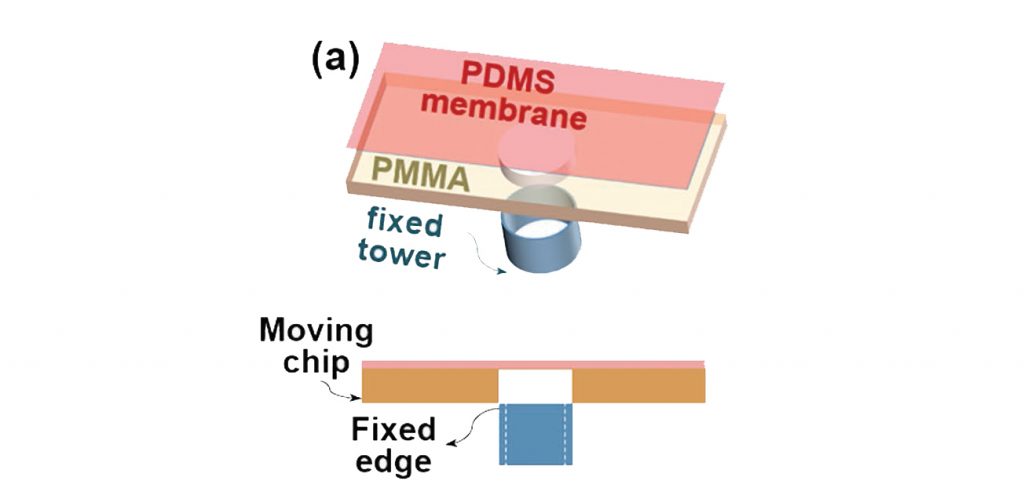
To print, cell-loaded bioinks are washed through the channels of the chip. In the circular center, a light flashes to cure a layer of material in the desired shape. As in SLA, the plate i.e. the center of the chip, moves down to make way for a new layer on top of the first one. Gradually, the inks build-up in successive layers into a 3D sample.
So far, the technique has been proved to print with up to 5 materials, including a liquid that serves to flush out unwanted material. As a proof of concept, the researchers bioprinted “tree-like” vein structures as seen in the image below.
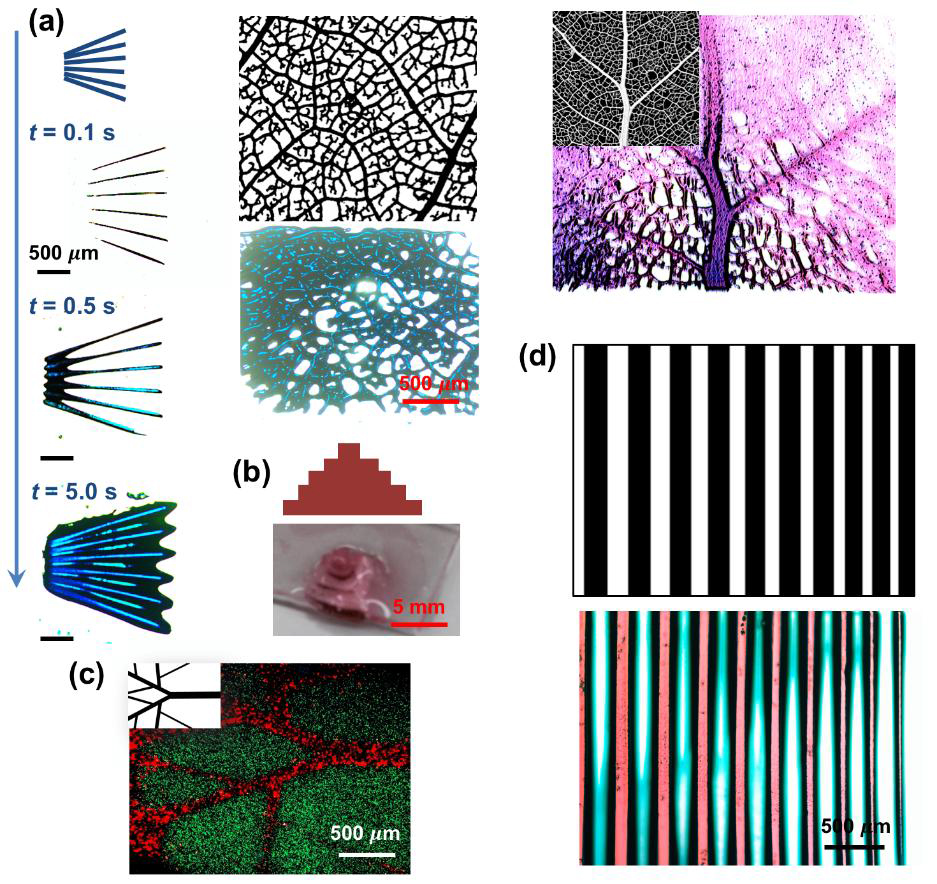
The need for speed
While there are many projects examining ways to recreate the complex, multimaterial structure of natural tissues, some are still hindered by the slow deposition rate of material extrusion technology. In this instance, the UCLA/HMS method finds it’s niche.
Zhang explains “The advantage [of our technique] mainly lies in the speed. Since SLA is based on layer-by-layer printing from a reservoir, it can crosslink a large area at a time making the speed faster than extrusion printing.”
In the work, the team also used a digital micromirror (DMD) device for digital mask generation, making the 3D printing process “even faster.”
The second advantage of the SLA technique is resolution, which is typically higher than extrusion-based technologies.
As standard, the UCLA/HMS bioprinter operates using UV light, which can incur some minor damages to living cells. However, Zhang asserts “Short time UV exposures are usually okay for the cells […] We can also switch to visible light photoinitiators to avoid the use of UV light.”
“Complex tissues” at “unprecedented ease”
Even at this rudimentary stage, results show that “The technology can facilitate fabrication of complex tissues potentially at unprecedented ease,” says Zhang. And, “With multi-material capacity it makes it possible to generate tissues with multiple types of cells and extracellular matrix molecules in a structurally biomimetic manner leading to better functions.”
Zhang and the team are optimistic that the technology could one day be used to make patient-specific tissue samples to be used in regenerative medicine, though the next step would be to scale the process. Project lead Khademhosseini has also previously led research into a microfludic platform to 3D print liver tissue.
“Microfluidics‐Enabled Multimaterial Maskless Stereolithographic Bioprinting” is published online in Advanced Materials journal. It is co-authored by Amir K. Miri, Daniel Nieto, Luis Iglesias, Hossein Goodarzi Hosseinabadi, Sushila Maharjan, Guillermo U. Ruiz‐Esparza, Parastoo Khoshakhlagh, Amir Manbachi, Mehmet Remzi Dokmeci, Shaochen Chen, Su Ryon Shin, Yu Shrike Zhang and Ali Khademhosseini.
Subscribe to the 3D Printing Industry newsletter, follow us on Twitter and like us on Facebook for exclusive interviews and all the latest cutting edge research.
Post and find new 3D printing jobs near you now.
Featured image shows Advanced Materials cover artwork commissioned to illustrate the SLA based microfluidic 3D bioprinting process developed by UCLA and HMS researchers. Image via Yu Shrike Zhang



