Bioprinting is coming on in leaps and bounds, but there are still issues printing thick vascular tissue. Now a team at Harvard University has revealed a new technique that promises to overcome the major problems using stem cells that essentially grow into the structure.
Anything beyond 1cm currently represents a serious challenge thanks to the added complexity of the extracellular gel matrix, vascular system it requires and the varying types of musculature.
The sheer complexity of these tissues, together with the challenge of providing a working vascular system that actually oxygenates and provides the various cells with nutrients to survive and thrive, was a real hurdle.
Harvard holds the key?
Now the team of scientists led by David Kolesky at the Wyss Institute for Biologically Inspired Engineering at Harvard University claim to have overcome the issue and created tissue above and beyond 1cm in thickness that they kept alive and well for more than six weeks.
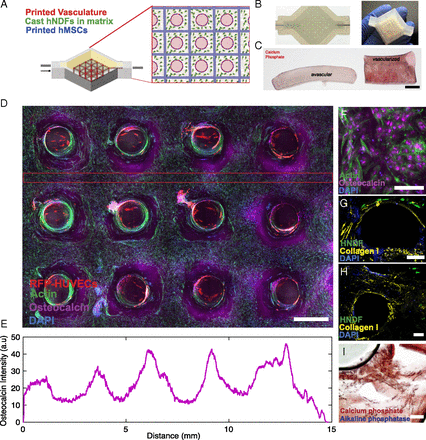
They also constructed tissues that integrated the basic building blocks of really human tissue: parenchyma, stroma and endothelial cells. The team achieved this by using human mesenchymal stem cells and neonatal dermal fibroblasts, contained within a custom printed extracellular matrix containing a proprietary vascular system lined with umbilical vein endothelial cells.
The team then essentially force fed the tissue through a perfusion chip to make them differentiate and grow into functional tissue. This creates an osteogenic lineage.
A path to organ transplants
To make genuine advances in human tissue engineering and to create organs, which could change the whole transplant scene, we have to be able to grow cells, extracellular matrixes and vascular systems in a controlled fashion. If we can do that to any level then we can make major advances with 3D cell cultures, drug screening, disease modelling and basic tissue repair.
Full organ creation lies at the end of that particular road. Actually building complex tissues has a path then and will get increasingly complex until we can simply print a new heart or liver for someone suffering from a life-threatening disease.
Capillaries are complex beasts
Perfusion, delivering blood through capillaries, has proved one of the biggest challenges to the process since day one. These are fragile vessels that are highly complex. Nutrients and oxygen must flow out, while waste products must be carried away. Replicating this through artificial means is a gargantuan task.
It requires biological, fugitive and elastomeric inks and a multimaterial design that runs right through the system. This is not a solid object you can print layer by layer and weld together with a laser.
Welcome to the matrix
The call-laden inks required a gelatin-fibrin matrix cross-linked by enzymes and the gelatin-fibrin matrix had to be thermally reversible in order to print it in the first place without polymerizing.
The castable matrix had to contain thrombin and TG, though, which would then diffuse through the structure to allow for long-term perfusion. It all had to happen without UV curing, too, as this process simply does not penetrate thick tissues and that has been one of the stumbling blocks for building deep bioprints.
The vascular system was created symmetrically and effectively endothelial cells were laid down in channels in the overall matrix. Honestly it is oversimplifying their work and does not do justice to the ingenuity on show.
It is intense science, but the short version is that we just took a big step towards bioprinting major organs and complex tissue that lasts for research and outright medical applications. This is the growth of human tissue, from base elements into a genuine growing organism. We can’t quite call it creating life, but it’s close.