Sols Systems, manufacturer of 3D printed orthotics, is set to be a leading player in the 3D printed footwear market. Co-founded by Kegan Schouwenburg who is also the CEO (and was formerly Director of Industrial Engineering and Operations at Shapeways) the company is already rolling in investment capital. In addition to a seed round of funding totalling $1.75 million, in December, the company recently announced a total of $6.4 million in funding from Lux Capital, with participation from Founders Fund, Rothenberg Ventures, Felicis Ventures, FundersGuild and Grape Arbor VC.
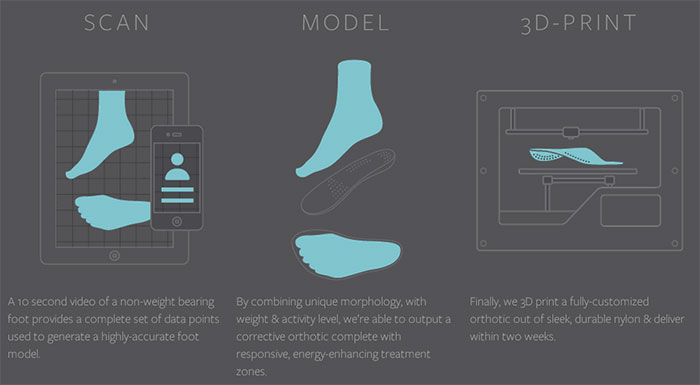
Orthopedic doctors can run Sols in their offices, using an iPad app to scan the feet of their patients. The company, then, 3D prints a custom insole, reducing the time and the price seen by traditional insoles. So far, Sols is beta testing the program with 50 doctors, shipping about 500 insoles. Doctors can set their own prices, with a suggested price of $500, set by Sols. In order to expand the product to consumers, the company will be launching an ‘over-the-counter’ insole for about $100 in 2015.
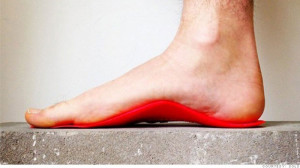
3D printing orthotics is a simple, but effective, way to demonstrate the power that 3D printing has to enhance the production of clothing and medical devices. Without getting into the complex, possibly uncomfortable realm of 3D-printed clothing or shoes, Sols can use the technology to make simple objects tailored to individual clients. Simultaneously, the company avoids the FDA guidelines necessary for more advanced medical devices. They can, then, produce a custom product that improves the life of a client as a proof-of-concept for what the technology can do for our lives as a whole.
Source: CNN Money