Researchers from the Karlsruhe Institute of Technology (KIT), Heidelberg University, and Osaka University, have developed a method to simultaneously stretch large numbers of single cells in order to study their mechanoresponsive behavior.
The scientists used 3D laser lithography to print stimuli-responsive composite scaffolds capable of stretching individual human bone tumor cells and embryonic mouse cells within tailored 3D microenvironments, to see how the cells are regulated by the mechanical properties of their surroundings.
“The broader focus of this work is to study the mechanotransduction of cells – to answer the question how cells respond to external mechanical signals such as compression or tension,” said Dr. Kai Weißenbruch, of the Zoological Institute and Institute of Functional Interfaces at KIT. “Understanding the molecular mechanisms of how cells respond to external stimuli is crucial, because these mechanical signals have a drastic influence on basic cellular processes such as tissue development, wound healing, and regeneration processes, but also during metastasis in the context of cancer cells.”
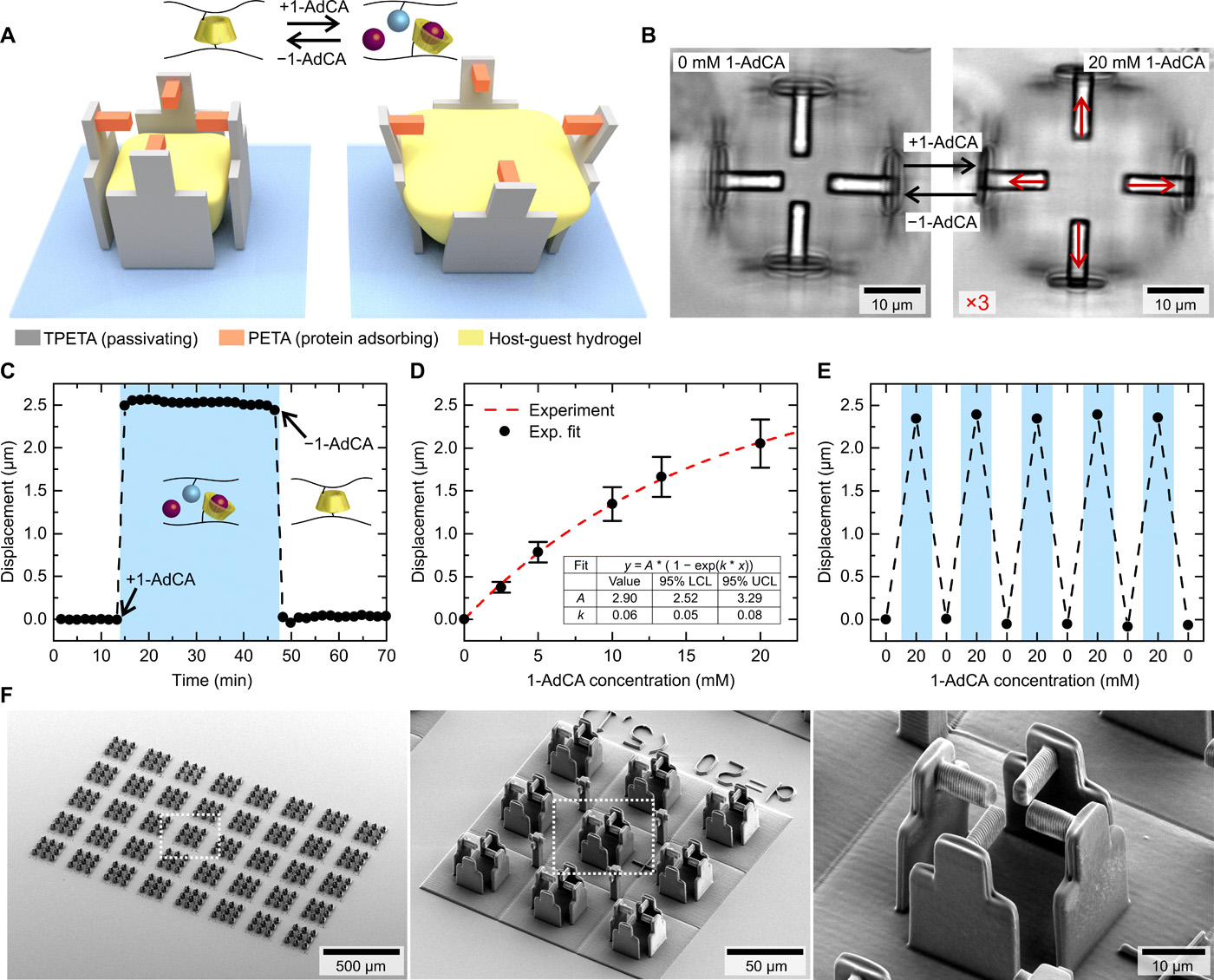
Creating the 3D printed ‘stretching rack’
The researchers began by developing a stimuli-responsive hydrogel suitable for use within the laser lithography 3D printing process. The two main components of the hydrogel were β-cyclodextrin acrylamide (βCD-AAm) and adamantane acrylamide (Ad-AAm), which form a host-guest complex in water. Adding or removing adamantane molecules to the solution gave the researchers on-demand control over the stimuli-response of the hydrogel once printed.
To manipulate single cells in a 3D environment, the researchers had to first guide them to the desired positions in the scaffolds and then apply a controlled external stimulus.
To produce the micro-scaffolds, within which the hydrogel is positioned, the researchers 3D printed four walls from a protein-repellent photoresist. Four 3D printed beams were then positioned on top of the walls, printed using a protein-attracting photoresist in order to guide the adhesion of cells in the scaffold.
By adding 1-adamantane carboxylic acid (1-AdCA) to the solution, the hydrogel expands and pushes on the micro-scaffold walls, which in turn bend and pull the four beams away from the center. A cell attached to these beams will therefore be ‘stretched’ from each side. Significantly, this process is completely reversible when washed out with pure phosphate-buffered saline (PBS).
“We combined two conventional materials with so-called stimuli-responsive or smart materials, which change their properties as a response to an external stimulation,” explained Marc Hippler, of the Institute of Applied Physics and the Zoological Institute at KIT. “The specific materials we used were developed by the group of Professor Akira Harada at Osaka University and we adapted them for the application in 3D laser lithography. This whole project was enabled by a close collaboration of physicists, chemists, and biologists.”
According to Hippler, 3D laser lithography was the only method that allowed the researchers to realize this specific micro-scaffold design.
“We required real 3D geometries with feature-sizes in the sub-micrometer regime, which is inaccessible by most other 3D printing techniques,” he continued. “Furthermore, the method readily enabled us to combine the three different materials which were required to construct the composite scaffolds to stretch individual cells.”
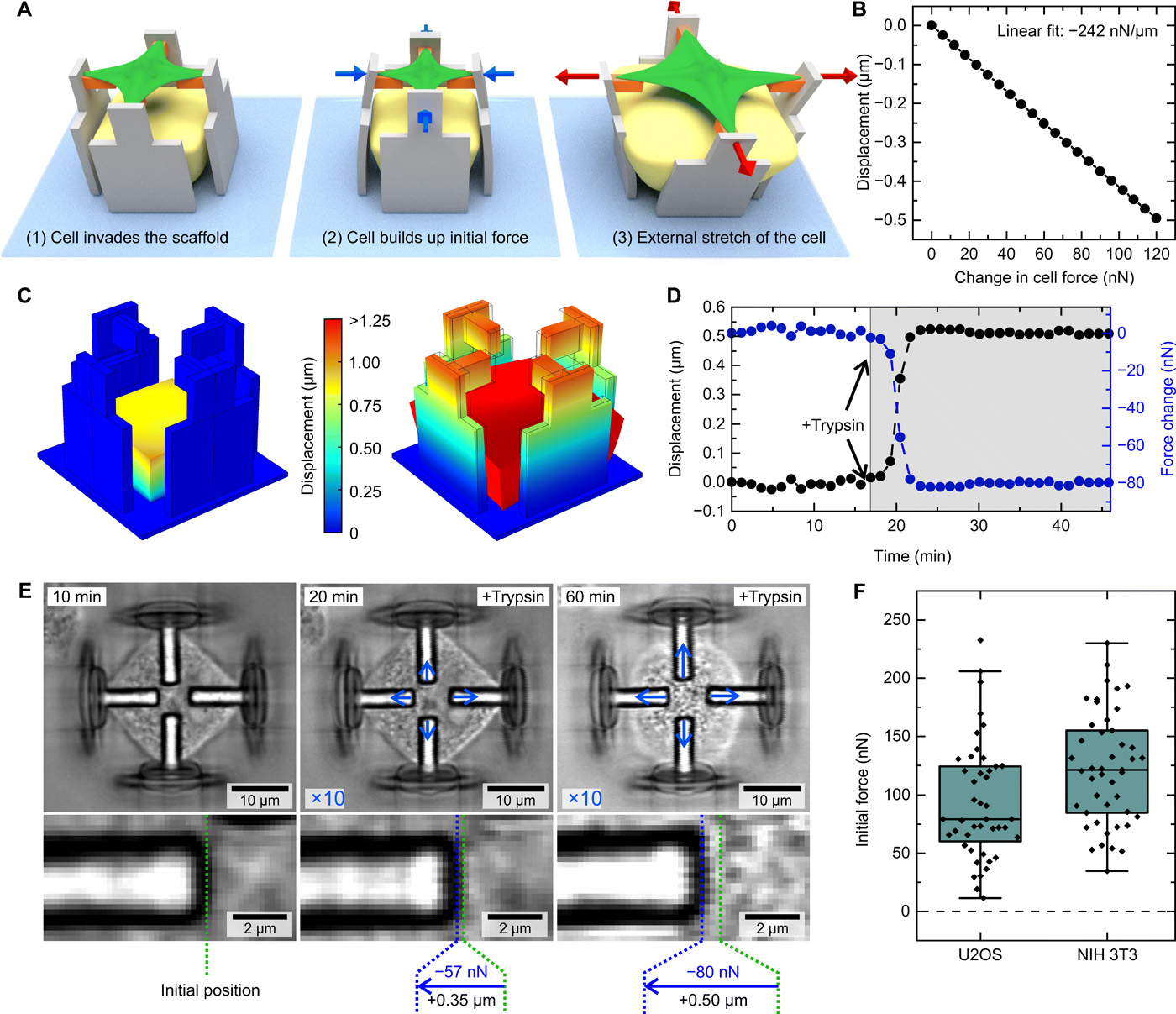
Investigating mechanotransduction
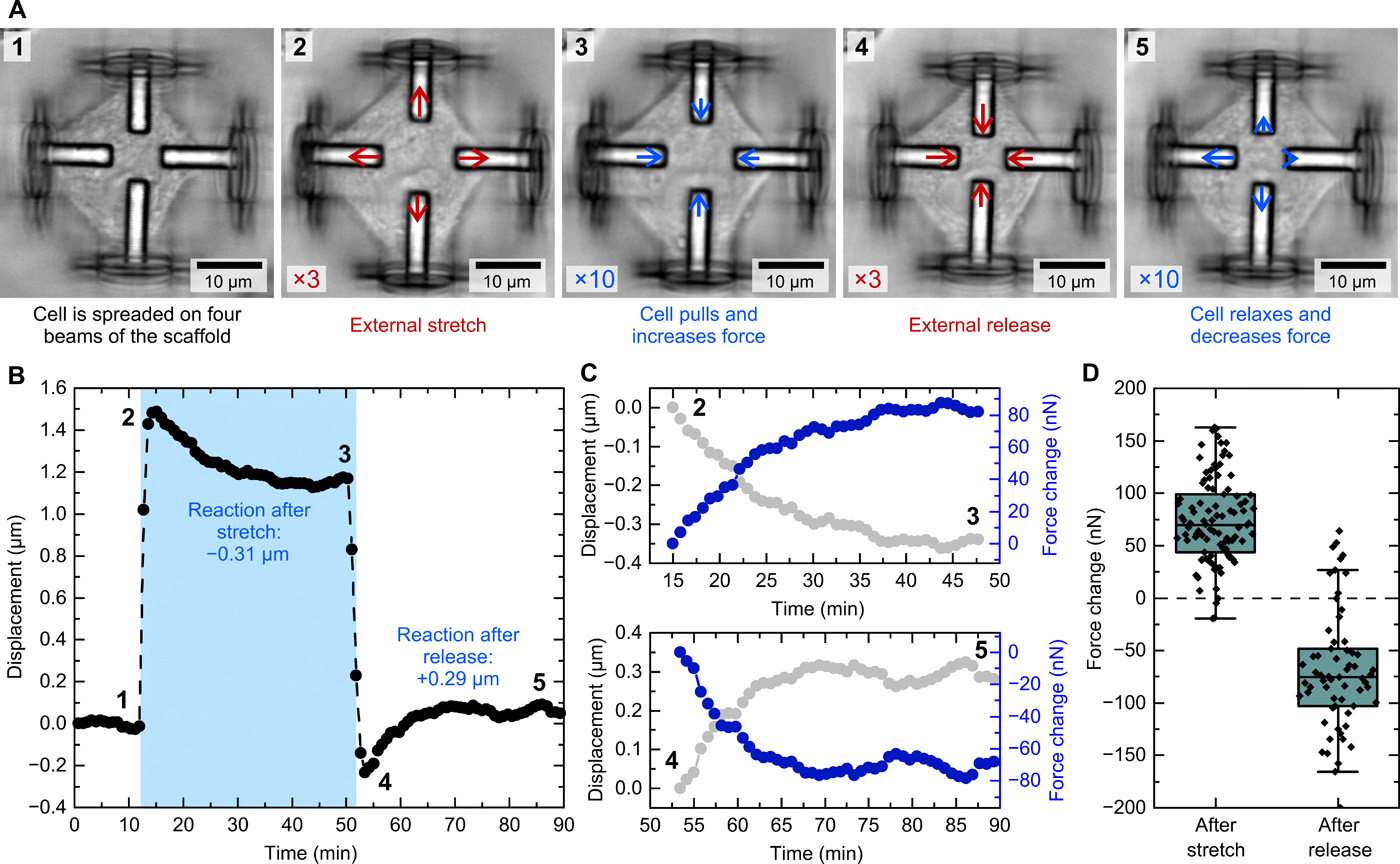
To monitor the displacement of the beams during the study, the researchers recorded a time series and carried out a digital image correlation analysis on the images. This allowed them to accurately track even the tiniest of cellular responses during the stretching process.
The cells chosen for use within the study were human bone tumor cells and embryonic mouse cells, selected for their contrasting properties.
“For the initial experiments in this study, we used to fundamentally different cell types, fibroblasts and epithelial-like cells,” said Weißenbruch. “Both are established model systems to investigate the mechanoresponsive behavior of cells. In this way, we were able to show that both cell types generate the same response to the external stimulation, thus validating that our observations are general cellular features during mechanotransduction.
“Our method can be readily applied to any adherent cell type which developed contact sites with its environment.”
Mechanotransduction refers to how cells sense and respond to mechanical stimuli by converting them to biochemical signals that evoke specific cellular responses. According to Professor Martin Bastmeyer, of the Zoological Institute and Institute of Functional Interfaces at KIT, this study forms only the beginning of the institute’s research into mechanoresponsive cells.
“In the present study, we introduced the basic mechanism of our method along with selected examples of its application,” he said. “Next, we want to investigate the underlying molecular mechanisms and intracellular processes responsible for the control and development of mechanotransduction.”
Further details of the study can be found in the article titled “Mechanical stimulation of single cells by reversible host-guest interactions in 3D microscaffolds“, published in the Science Advances journal. The article is co-authored by M. Hippler, K. Weißenbruch, K. Richler, E. Lemma, M. Nakahata, B. Richter, C. Barner-Kowollik, Y. Takashima, A. Harada, E. Blasco, M. Wegener, M. Tanaka, and M. Bastmeyer.
3D printing micro-scaffolds
In recent years, 3D printing has been utilized in the manufacturing of micro-scaffolds for several applications, including tissue engineering research and various medical applications.
In particular, research into micro-scaffolds has become increasingly centered around regenerative medicine. In 2017, research institutions in Brazil combined two 3D printable polymers into cell scaffold structures ideal for cell-growth, while research conducted by Korea Polytechnic University and Gachon University in South Korea demonstrated the use of Blu-Ray powered micro-stereolithography (MSTL) to make scaffolds suitable for the regeneration of bone cells.
More recently, 3D printed micro-scaffolds have been used to support bone regeneration in horses, and could even be capable of fighting off cancer cells.
Nominations for the 2020 3D Printing Industry Awards are still open, let us know who is leading the industry now.
The fourth edition of the 3D Printing Industry Awards Trophy Design Competition is now underway. Enter your design for the chance to win a CraftBot Flow 3D printer.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows schematic images of a cell that first invades the scaffold, builds up initial force in the process, and is later stretched by the scaffold. Image via Science Advances.



