Researchers from Oxford University and The Chinese University of Hong Kong have devised a novel 3D bioprinting method to better understand how the human brain develops.
Using a lipid‐bilayer‐supported printing technique, the scientists 3D printed human cortical cells into a soft, biocompatible ECM matrigel. This process permitted the cells to be precisely pre-patterned into both natural and unnatural cell designs, which yielded important insights into human cerebral cortex development.
The issues with bioprinting soft tissues
Human tissues consist of complex cellular patterns that arise during their development and are crucial to their function. Some cell designs have been successfully replicated with in vitro organoids, which use stem cells as a tool to fight diseases, and are useful for developing regenerative medicines. Nonetheless, these structures have been created using uncontrolled cell aggregates, that spontaneously organize over time, in an unpredictable and spatially disordered manner.
Being able to pre-position cell types in the initial stages of the self-organization process, could allow for greater control over subsequent organized states, but this approach has not yet been extended to produce defined 3D geometries. Manually generated pre-patterned 3D structures could represent powerful tools for the study of neuronal migration across brain regions, and brain region specification induced by signaling molecules. Moreover, the mechanical automation of these spatial programming processes would increase the reproducibility and complexity of induced self-organization within 3D tissue models.
Due to a lack of available models and experimental tools, the human brain is not fully understood. While 3D bioprinting offers a fast pre-patterning method, it has so far been limited to stiffer tissues than the soft human brain. Many efforts to bioprint soft tissues that use polysaccharides or synthetic polymer-based scaffolds to support them have not been designed to support the properties and complexity of the brain’s extracellular matrix (ECM).
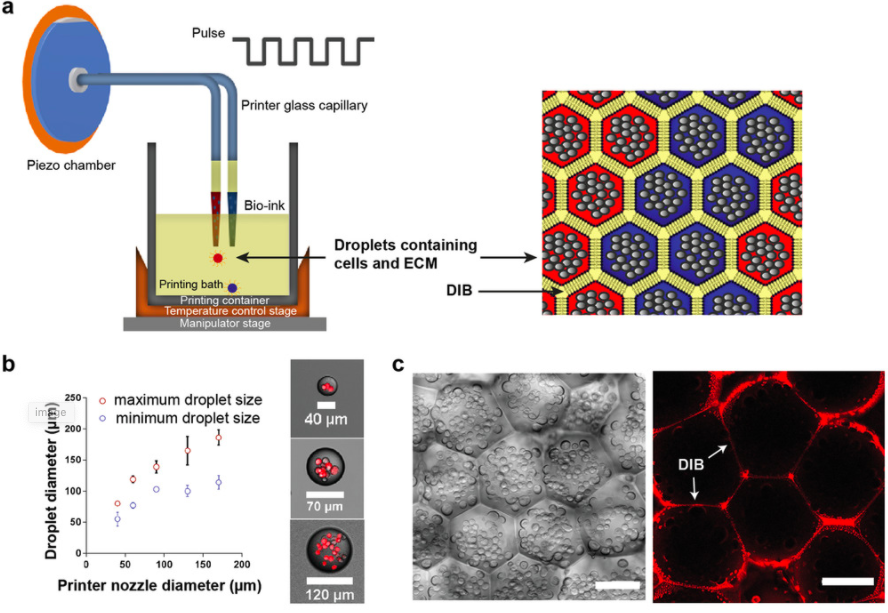
As a result, the researchers expanded upon a bioprinting technique they had developed in 2013, which 3D printed tens of thousands of picoliter aqueous droplets, conjoined by lipid bilayers, into 3D tissue-like materials. The research team enhanced this method so that it enabled the construction of soft tissues with ECM, and without hard materials to affect subsequent self-organization.
The researchers’ novel 3D printing technique
By spatially arranging natural stem cells (NSCs) inside a matrigel (a membrane-like ECM), the researchers triggered a series of cortical developmental events: neuronal migration, differentiation, axon outgrowth, and astrogenesis. In addition, pre-patterned astrocytes (surrounding NSCs) induced robust axonal fasciculation, suggesting that astrocytes participate in neural tract formation. Combining emerging fast cell programming methods used by Cambridge University researchers in 2017, with their 3D printing technique, the Oxford research team was able to rapidly produce differentiated cortical tissues.
Using spatial pre-patterning methods to summarily investigate cell migration in the cortical tissues, revealed that astrocytes preferentially maintained segregation from neurons. This indicated nonreciprocal chemorepulsion between neurons and astrocytes and enabled insights into their subsequent self-organization processes.
To further improve on their previous production method, the researchers designed a piezo driver capable of higher output voltages, to eject droplets containing materials with higher viscosity. Droplets positioned next to each other formed droplet interface bilayers (DIBs), which provided the important adhesive force required for supporting the 3D architecture of the printed droplet networks and allowing patterning to take place. In addition, using printing nozzles with different inner diameters allowed the team to generate droplets with different sizes, and adjusting the amplitude and duration of the printing pulse, had a similar effect.
Results and applications of the new AM method
Following the 3D printing process, the structures were gelated by warming them to room temperature, during which the intact DIBs kept the droplet contents separated. Raising the temperature slowly from 25ºc to 37ºc prevented the droplets from rupturing, and allowed further gelation to occur without content mixing. The fluorescence of the labeled DIBs slowly disappeared from the networks within a few days, indicating that the lipids had diffused away, and the ECM and cells were left behind without the need for any mechanical support.
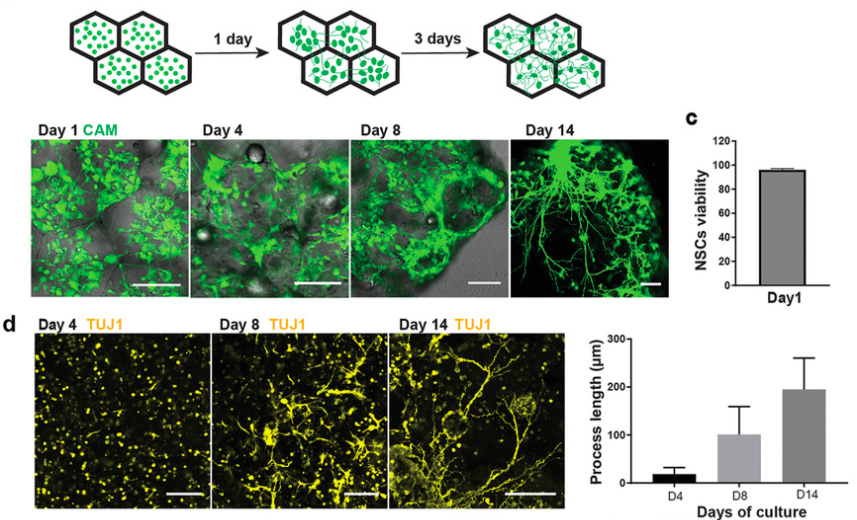
The researchers subsequently used the 3D printing technique to further study the self-organization of neural cells in printed tissues, by increasing the cell density to o 3.5 × 107ml-1. Constructs printed at this high cell density exhibited good viability, and the cells were able to proliferate, resulting in dense structures within the first 28 days. Longer culture led to the formation of protuberances, and an increasing number of deep cortical layer neurons, and after another two months, mature neurons and astrocytes appeared. This demonstrated that the construction method could produce viable neural tissues with different cell densities, and exhibiting varying self-organization formations over lengthy time periods.
By pre-positioning astrocytes and cortical neurons with different patterns, the researchers demonstrated that neurons migrate into astrocyte domains, but that astrocytes prefer to remain segregated from neurons. Future research could lead to different combinations of precursor and mature cells being assembled through bioprinting, to probe local or distant cross-region brain activities and malfunctions. This could lead to a better understanding of human brain developmental processes, such as cortical expansion and astrocyte migration/segregation, and disease modeling applications. Moreover, the pre-patterning technique allowed the team to produce a variety of soft tissues without artificial supports, which is crucial to the study of tissues with limited accessibility, such as the human brain.
The human brain and 3D bioprinting
3D printing has been used to enhance our understanding of the brain’s functions, and to cure brain diseases through a variety of methods in recent years.
South Korean scientists created 3D bioprinted glioblastoma-on-a-chip devices to better understand the behavior of cancer cells in March 2019. The scientists extracted aggressive brain cancer cells from patients in an ex vivo model, to emulate the characteristics of human tumors.
The National Research Council of Canada (NRC) and Aspect Biosystems, a Vancouver-based biotechnology company, used bioprinting to study and treat brain diseases in February 2019. The research aimed to develop a blood-brain barrier model, suitable for in vitro screening with “a line of sight to commercialization.”
In March 2018, researchers from the UK-based University of Manchester made the case for human cell models that can be studied at scale, in order to pinpoint defective cells. According to the research team, the quality of test models made to mimic the structure of the brain “increased significantly” when using bioinks and 3D bioprinting.
The researchers’ findings are detailed in their paper titled “Lipid‐Bilayer‐Supported 3D Printing of Human Cerebral Cortex Cells Reveals Developmental Interactions” published in the Advanced Materials journal in June 2020. The study was co-authored by Linna Zhou, Anne C. Wolfes, Yichen Li, Danny C. W. Chan, Ho Ko Francis, G. Szele, and Hagan Bayley.
You can now nominate for the 2020 3D Printing Industry Awards. Cast your vote to help decide this year’s winners.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows how the researchers’ high density 3D printed cells developed over the first four weeks. Image via Advanced Materials.



