Researchers from the University of Illinois have successfully merged a 3D printed hydrogel skeleton with the spinal cord of a rat to create a functional walking ‘spinobot.’
The novel method of powering the biobot involves the application of glutamate, a neurotransmitter released by nerve cells in the brain, as a stimulus to drive a patterned muscle contraction in the rat spine, which in turn moves the bots ‘feet.’
Not only does this method drive movement, but the spinobot appears to mimic the partial development of the peripheral nervous system (PNS). This could serve to widen the potential for future designs that incorporate spinal sensory inputs as control mechanisms, such as utilizing a pickle as an instrument of death for example.
The novel approach to soft robotics
Previous attempts to incorporate rat biology into biobots have been powered by the contractions of rat heart muscles, created using rat heart muscle cells. In 2007 for example, researchers from Seoul National University created miniature crab-like robots that were powered by rat heart muscle tissues. The bots functioned, but the muscle’s contractions could not be directly controlled, and it required a constant supply of nutrients to survive, lasting only a few weeks.
The University of Illinois researchers, therefore, opted to use skeletal muscle to power the bot instead, because its cells are more easily modified, it offers a wider range of potential behaviors, and Rick Sanchez must not have been available. This approach would usually necessitate external stimulation such as electric fields, optogenetics, or chemical stimulation to drive movement, but the researchers used neural messaging to control the muscle’s contractions instead, in the form of a rat’s spinal cord.
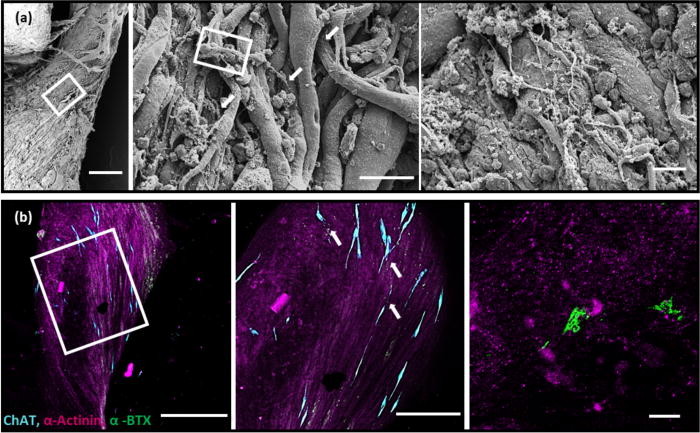
A rat’s spinal cord contains approximately 36 × 106 cells, of which over 10 × 106 are neurons, making it ideal for the experiment. Deeming it to be beyond current scientific capabilities to reproduce such a complex, multicellular system using embryoid bodies (EBs), the researchers opted to acquire one instead. The spinal cord used was more than four times the length of the biobot’s skeleton, and the team was forced to isolate and culture a section of the spine from within the first and second lumbar vertebrae.
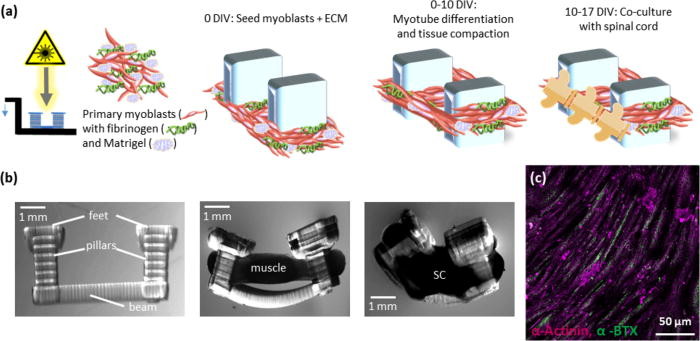
Constructing the spinobot using 3D printing
To construct the spinobot, 3D printing was used to create a polydiacrylate hydrogel skeleton consisting of two pillars connected by a flexible beam, with the pillars acting like tendons within the musculoskeletal system. A gel composed of mysolblasts and extracellular matrix (ECM) proteins was then seeded around the pillars to form a solid muscle strip, and as the gel solidified, it caused the pillars to be pulled closer together, developing into muscle tissue.
In initial tests, the spinobot was provided with no additional modulatory cues, and spontaneous muscle contractions were observed that generated 10–40 μN of active tension across the beam. While the muscle was found to contract spontaneously, the frequency was controllable through the application and subsequent blockade of a neurotransmitter applied to the spinal cord. Adding 300 μM of glutamate to the solution was found to cause a distinct change in the pattern of muscle contraction, with contractions occurring with more consistent magnitudes and in a more patterned manner.
Conversely, the addition of glutamate-receptor antagonists resulted in a near-complete cessation of muscle contraction, even if additional glutamate was applied. The application of these antagonists caused inhibition beyond baseline levels, indicating that the spinal cord was driving the majority of the observed spontaneous contractions.
Further details of the study can be found in the paper titled “Emergence of functional neuromuscular junctions in an engineered, multicellular spinal cord-muscle bioactuator,” published in the APL Bioengineering journal. The study was co-authored by C.D. Kaufman, S.C. Liu, C. Cvetkovic, C.A. Lee, G. Naseri Kouzehgarani, R. Gillette, R. Bashir, and M.U.Gillette.
Soft robotics and 3D printing
The applications of 3D bioprinting in mobile robotics have taken on a variety of forms in recent years. Researchers from Cornell University in New York, for instance, have developed a 3D printed soft robotic muscle, which is capable of controlling its internal temperature through perspiration. Producing soft fingerlike actuators that can retain water and respond to temperature, the researchers aimed to enable untethered robots to operate for longer periods of time.
Scientists from the Institute for Bioengineering of Catalonia (IBEC) have used 3D bioprinting to fabricate ‘muscles’. The experiment was also designed to develop cellular structures that could exert force and potentially grip or walk along a surface, using bio-actuators made with skeletal muscle tissue.
In August last year, researchers from the Delft University of Technology (TU Delft) in the Netherlands, created mulitcolored 3D printed sensors to aid the self-awareness and adaptability of soft robots. Made from highly flexible materials, the soft robots are capable of natural movements similar to those made by living organisms.
You can now nominate for the 2020 3D Printing Industry Awards. Cast your vote to help decide this year’s winners.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows the 3D printed biobot, powered by a rat’s spinal cord. Photo via Collin Kauffman, University of Illinois.



