Chinese pharmaceutical and 3D printing technology firm Triastek has received Investigational New Drug (IND) approval from the US Food and Drug Administration (FDA) for its first 3D printed drug product, T19.
T19 has been developed in-house and is designed to treat rheumatoid arthritis, which is an autoimmune disease where the body’s immune system attacks the cells that line joints by mistake, making them stiff and swollen.
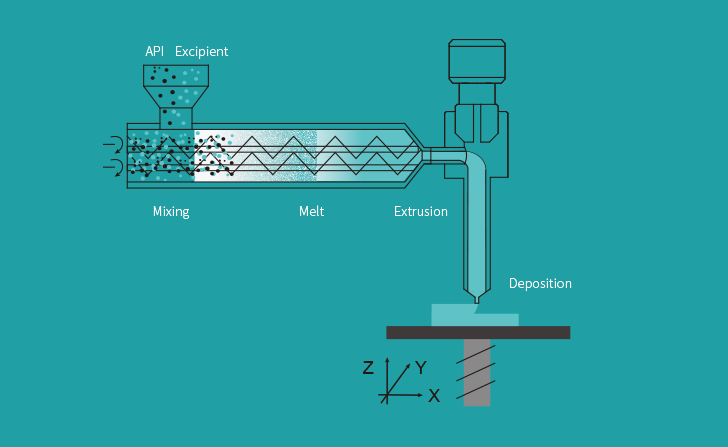
Triastek 3D printed the drug product using its Melt Extrusion Deposition (MED) technology platform and has global intellectual property (IP) rights to its 3D printed formulation.
“Triastek is committed to improving the efficiency of formulation development, enhancing the effects of drug products, and ensuring the quality of drug delivered to patients by using the 3D printing technology platform,” said Dr. Senping Cheng, Co-founder and CEO of Triastek. “The FDA IND clearance of T19 is an important milestone in the development and application of MED 3D printing technology.”

MED 3D printing platform
Triastek’s MED 3D printing technology platform uses digital pharmaceutical dosage form design and automated intelligent manufacturing to enable the construction of tablets with sophisticated shapes and internal geometric structures. These structures allow the onset time, duration, and mode of drug delivery to be closely controlled and adjusted, which in turn provides more predictable and reproducible drug delivery results.
By enabling greater customization of the printed drugs, the MED system can help to enhance the therapeutic effects of medicines, while lowering side effects and improving compliance with patients. Triastek’s novel pharmaceutical product development method, 3D formulation by Design (3DFbD) can be used in tandem with MED 3D printing technology to reduce the need for trial and error often required during traditional formulation development processes. 3DFbD is designed to improve both the efficiency and success rate of Triastek’s drug product development in order to accelerate development time and lower costs.
The firm has also integrated real-time Process Analytical Technology (PAT) into the MED system that can continually monitor the 3D printing process to ensure product quality and make regulatory monitoring more convenient.
In April last year, the MED platform was accepted into the FDA’s Emerging Technology Program (ETP) which recognized the 3D printing technology as a fully automated process for the manufacture of modified release solid oral dosage forms.
“We believe that the MED 3D printing technology will be the enabler for digital pharmaceutical product development and intelligent drug manufacturing,” said Dr. Xiaoling Li, Triastek’s Co-founder and CSO. “Triastek will work with any interested parties to take advantage of the platform technology for developing pharmaceutical products with better clinical value and higher product quality.”
A chronotherapeutic drug delivery system
According to Triastek, deploying 3D printing for T19’s novel design permits it to function as a chronotherapeutic drug delivery system. Chronotherapy treatment is based on the idea that administering medicine at different times of the circadian cycle – the body’s natural internal process that regulates sleep over a 24 hour period – will maximize a drug’s therapeutic impact while minimizing side effects.
The T19 tablet is 3D printed on Triastek’s MED system in a specific shape and with a precise internal geometric structure that facilitates the precise control of the drug’s release to achieve the desired uptake by the patient. Taken at bedtime, T19’s design means the drug will be released in a delayed manner so that blood concentration peaks when symptoms of pain and joint stiffness are most acute, such as in the early hours of the morning.
Having received IND clearance from the FDA for T19, Triastek is planning to apply for the same approval in China later this year, followed by applications in Japan and Europe. The firm expects to file a New Drug Application (NDA) for T19 to the FDA in 2023, and announced it has also developed a 505(b)(2) product portfolio using MED 3D printing technology to improve the outcomes of drug therapy.

3D printing and drug delivery
Personalized medication for individual patients is a fast-growing area of interest within the 3D printed pharmaceutical field. 3D printing enables the size and geometries of tablets and drug delivery devices to be modified to control their release and dosages depending on clinical need.
In 2015, Aprecia Pharmaceuticals received FDA approval for its 3D printed Spiritam medication, making it the first 3D printed pharmaceutical to be approved by the FDA. The medication is designed to treat seizures in people with epilepsy and was approved for commercial manufacturing operations later that year. Aprecia recently announced a long-term collaboration with R&D firm Battelle to expand its capabilities within 3D printed pharmaceuticals and advance its 3D printing equipment from clinical supply to commercial scale. The firm has also previously partnered with Cambridge-based Cycle Pharmaceuticals to produce orphan drugs for the treatment of rare medical conditions using its ZipDose 3D printing process.
3D printed pharmaceuticals firm FabRx has also been active on the printed drug development front, having produced personalized medicine for children with the rare metabolic disorder maple syrup urine disease (MSUD), and printed its chewable Printlet tablets with Braille and Moon patterns on the surface to aid medicine taking for patients with visual impairment. The company launched its M3DIMAKER 3D printer in April 2020, designed specifically for the manufacture of personalized drug delivery devices.
Elsewhere, global pharmaceutical firm Merck has announced plans to work with EOS Group company ACMC to produce 3D printed tablets first for clinical trials, then later for commercial manufacturing. Meanwhile, in the research realm 3D printing has been used to optimize the controllable dosage of antibiotic tablets, and semi-solid extrusion 3D printing has been explored as a coating technology for customizing the release rate of patient-specific drugs.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows Triastek has gained IND clearance from the FDA for its T19 drug for rheumatoid arthritis. Image via Triastek.



