Researchers from Harvard University’s Wyss Institute have developed a suite of new heart engineering technologies that have enabled them to mimic the alignment of a human heart’s contractile elements.
Leveraging a bioink containing contractile organ building blocks (OBBs) and patient-specific stem cells, the team was able to bioprint sheets of cardiac tissue with complex and varied alignments similar to those in actual human heart muscle layers.
In the future, the team’s advance could enable the development of thick multi-layered human muscle tissue with more physiological contractile properties and, potentially, pave the way toward 3D printed heart tissue replacements.
“Being able to effectively mimic the alignment of the heart’s contractile system across its entire hierarchy from individual cells to thicker cardiac tissue composed of multiple layers is central to generating functional heart tissue for replacement therapy,” said Jennifer Lewis, senior author of the study and Wyss Core Faculty member.
The SWIFT bioprinting platform
The Wyss Institute has been developing innovative bioprinting technologies and tissue engineering techniques for a number of years. For instance, in February, bioengineering start-up Trestle Biotherapeutics licensed a Harvard University-developed technology that enables the rapid fabrication of kidney tissues at scale.
The team has been looking into bioprinting heart tissue for some time, having created a 3D printing workflow to predict the performance of artificial heart valves back in 2018. A year later, the scientists developed their novel sacrificial ink-writing technique called SWIFT (sacrificial writing into functional tissue) to 3D print large, vascularized human OBBs.
The team was able to create cardiac tissue that fuses and beats synchronously over a seven-day period, and has expanded upon this achievement in its latest study.
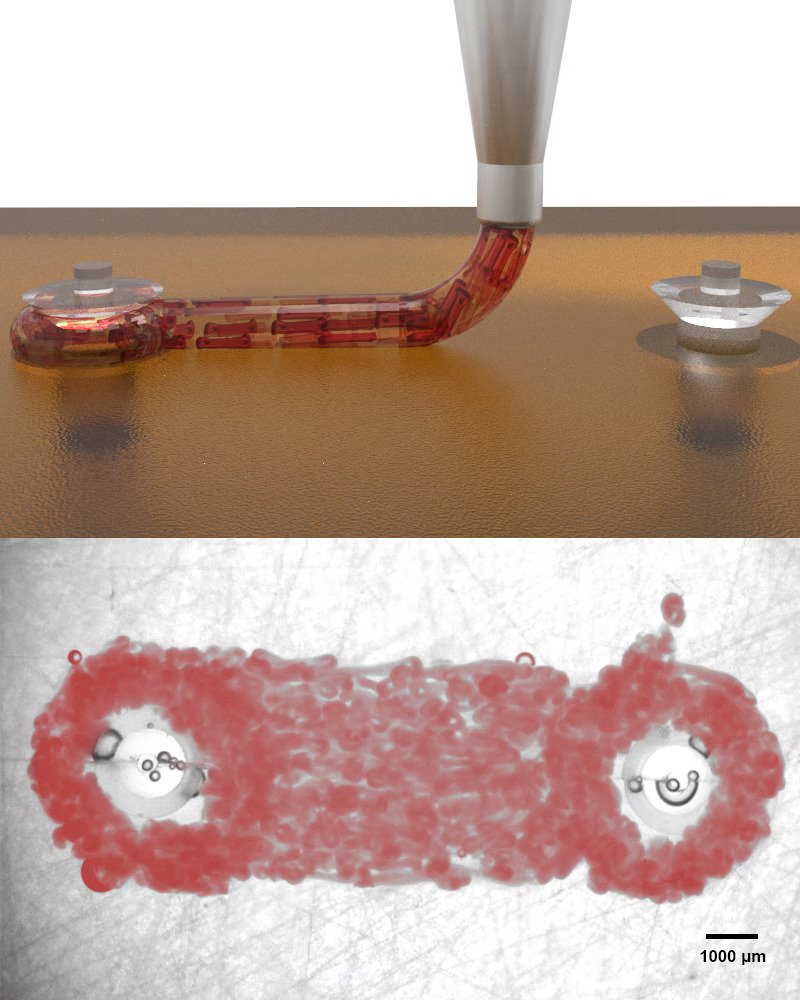
Bioprinting human heart muscle
The Wyss Institute team’s latest study builds on its SWIFT bioprinting platform. The approach makes use of preassembled OBBs made from human induced pluripotent stem cells (hiPSCs-CMs) to produce blood-supporting vascular networks using sacrificial inks. Using the platform’s sophisticated 3D bioprinting capabilities, the scientists were able to create cardiac tissue constructs that have the typical high cellular densities of normal heart tissue.
“To also gain control over directional contractility in engineered layers of heart tissue, we first devised a strategy to program the parallel alignment of iPSC-CMs in developing OBBs,” said first-author John Ahrens, who is a graduate student in Lewis’ group.
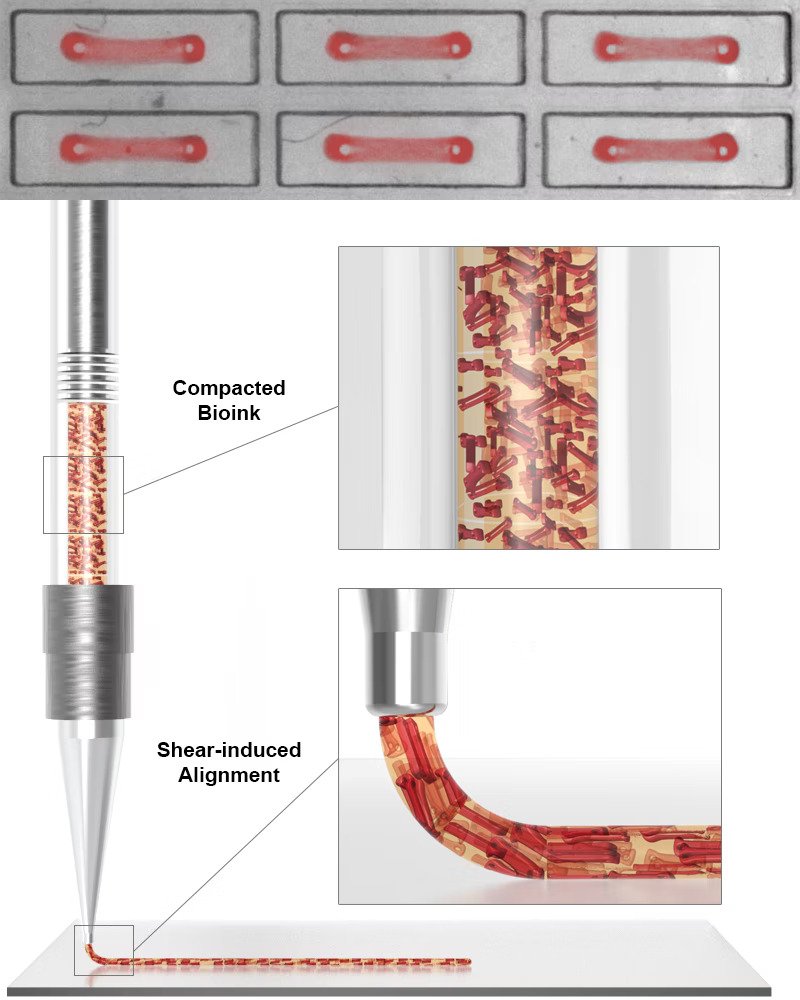
To create the cell-laden bioink, the team developed a platform with 1050 wells that each contain two micropillars. They then seeded hiPSCs-CMs into the wells via a mixture containing human fibroblast cells and the extracellular matrix (ECM) protein collagen, both of which are essential for heart muscle development.
As the cells compact the ECM, they formed a dense microtissue within which the cardiomyocytes and their cellular contractile machineries were oriented along the axis connecting the micropillars. The OBBs, capable of contracting in one major direction, were then lifted off the micropillars and used as feedstock for the bioprinting process.
“Our lab has previously shown that it was possible to align anisotropic soft materials via 3D printing,” said study co-author Sebastien Uzel. “Here, we demonstrated that this principle could be applied to cardiac microtissues too.”
To demonstrate their technique, the scientists 3D bioprinted cardiac tissue sheets with linear, spiral, and chevron geometries within which the OBBs showed significant alignment.
Evaluating the 3D printed heart muscle
Once the OBB-laden cardiac tissue layers had been bioprinted, the team sought to measure the contractile features of the constructs. The researchers 3D printed long macrofilaments connecting two macropillars, in a similar but larger-scale approach to the OBB-generating step during the bioink formulation process.
The team was able to measure the macropillar deflections to determine the contractile forces generated by the macrofilaments, and found that the tissues’ contractile forces and contraction speed increased over a seven-day period. Simply put, the 3D printed tissues continued to mature into actual muscle-like filaments.
“With SWIFT, we wanted to address cellular density and tissue scale,” said Uzel. “Now, by programming alignment, we aimed for mimicking the microarchitecture of the myocardium. One innovation at a time, we are moving closer to engineering functional cardiac tissues for repair or replacement.”
Going forwards, the team is planning to apply their method to fabricate more physiological tissues beyond single-layered constructs.
“While the holy grail of tissue engineering efforts would be a whole organ heart transplantation, our approach could enable contributions to more immediate applications,” added Ahrens. “It could be used to generate more physiological disease models, and create highly architected myocardial patches that, like LEGO blocks, could match and be used to replace a patient-specific scar after a heart attack. Similarly, they could be tailored to patch up patient-specific holes in the heart of newborns with congenital heart defects.
“In theory, these patches could also develop with the child and not have to be replaced as the child grows.”
Further information on the study can be found in the paper titled: “Programming cellular alignment in engineered cardiac tissue via bioprinting anisotropic organ building blocks,” published in the Advanced Materials journal. The study is co-authored by J. Ahrens, S. Uzel, M. Skylar-Scott, M. Mata, A. Lu, K. Kroll, and J. Lewis.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews, and webinar replays.
Featured image shows bioprinting long cardiac macrofilaments to measure the macropillar deflections caused by the contractile heart muscle layer. Image via Harvard University.



