In yet another big step toward the development of functional 3D printed “bioficial” cartilage for use in humans, scientists at University of Melbourne have shown that infrapatellar fat pad adipose stem cells (IPFP-ASCs) can leverage 3D printed scaffolds to aid cartilage repair by developing a “cap” of cartilaginous tissue on top of the scaffold itself.
Cartilage is a sort of “holy grail” for bioprinting, as it is one of the most likely candidates to be the first biopprinted tissue for the repair of damage done to cartilaginous structures of the knee and veterbrae, as well as the ears and trachea. Cartilage related pathologies are extremely common and can result in pain or loss of function for the patient, often degenerating into osteoarthritis of the joint. The adult body is not capable of fully self-repairing its cartilage. Due to its layered, single-cellular structure, however, cartilage may be 3D printed effectively.
Finding the right consistency (not too hard and not too soft) remains a challenge, but the combination of cells, scaffold and biochemical factors may provide the possibility of true cartilage regeneration. IPFP-ASCs have been shown to harbor chondrogenic potential, that is the potential to develop into cartilaginous tissue. When combined with 3D polymeric structures, the stem cells were successfully used in the lab to provide a source of stem cells to engineer 3D tissues for cartilage repair.
In this PLOSONE published study, the researchers, funded by various Australian medical research institutions, have shown that human IPFP-ASCs seeded onto 3D printed chitosan (a polysaccharide widely used in tissue engineering) scaffolds can undergo chondrogenesis using TGFβ3 and BMP6, growth factors naturally involved in cell differentiation, embryogenesis, and development.
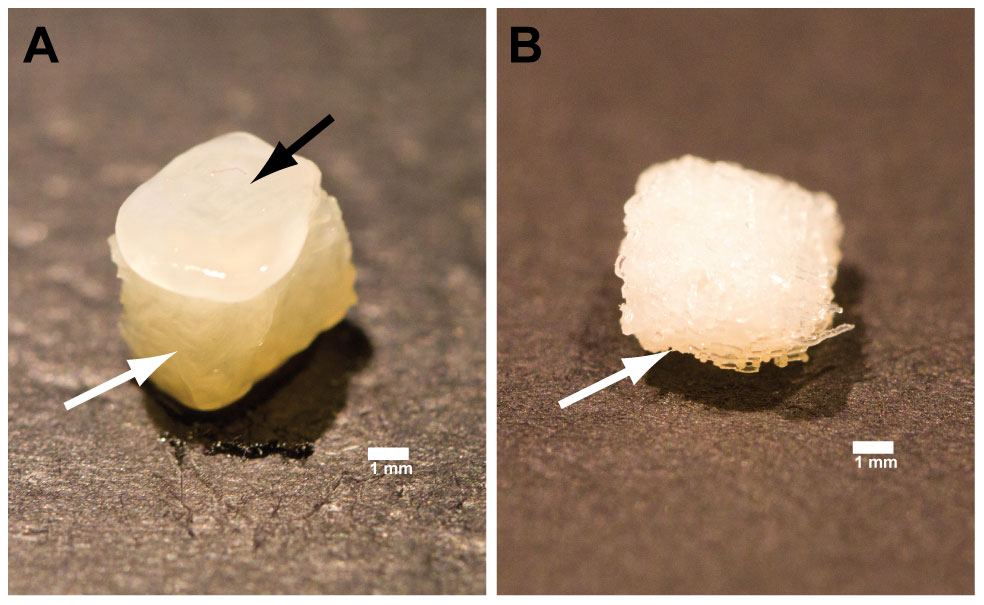
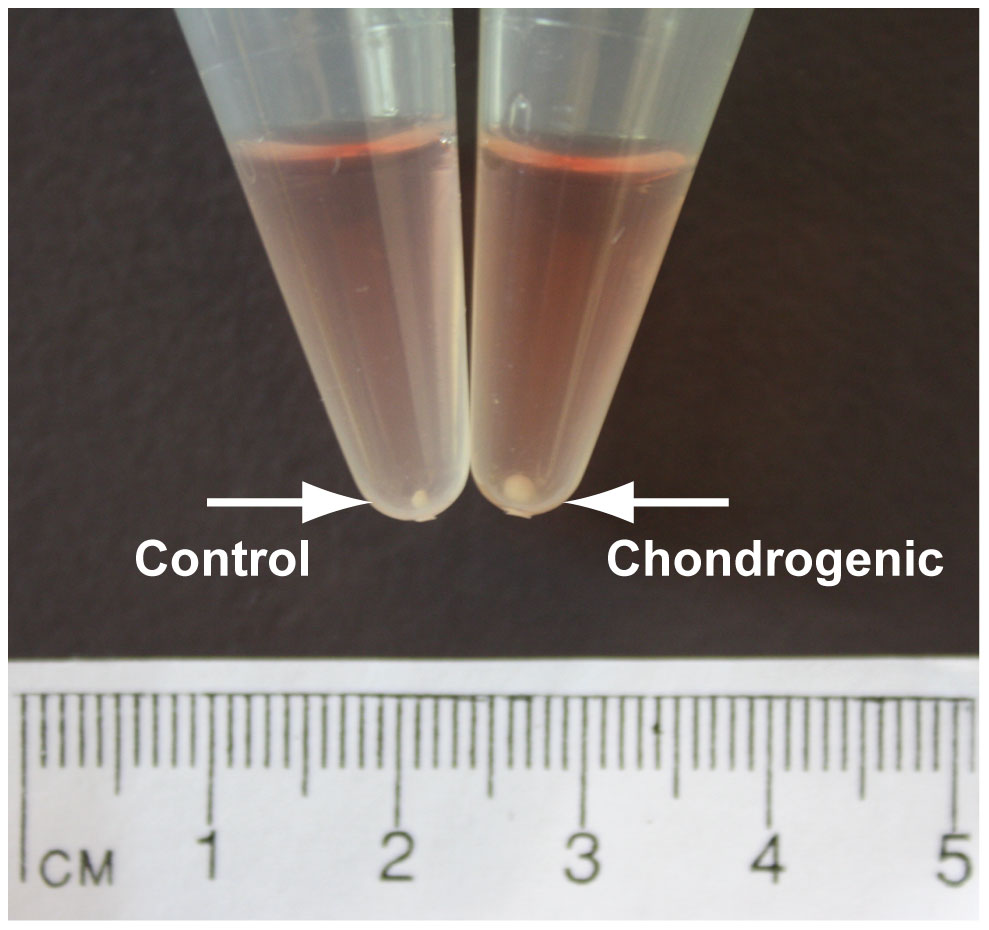
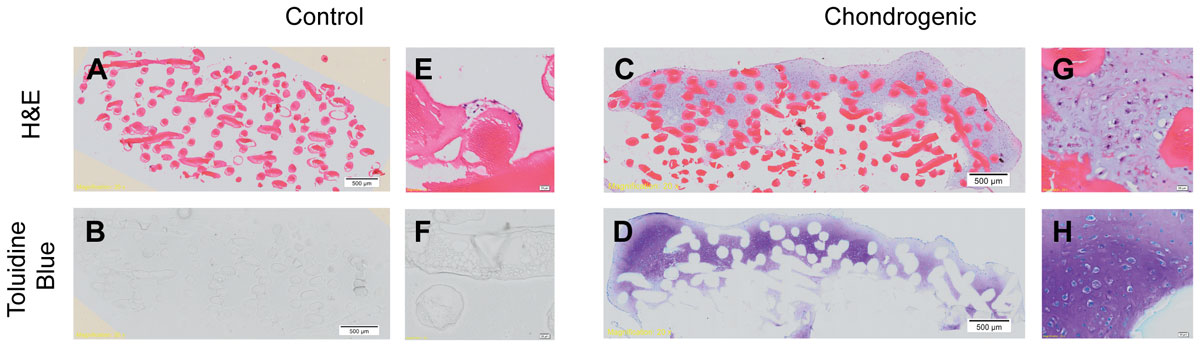
By week 4, a pearlescent, cartilage-like matrix had formed that penetrated the top layers of the chitosan scaffold, forming a ‘cap’ on the scaffold. Chondrocytic morphology showed typical cells encased in an extracellular matrix, stained positively with toluidine blue.
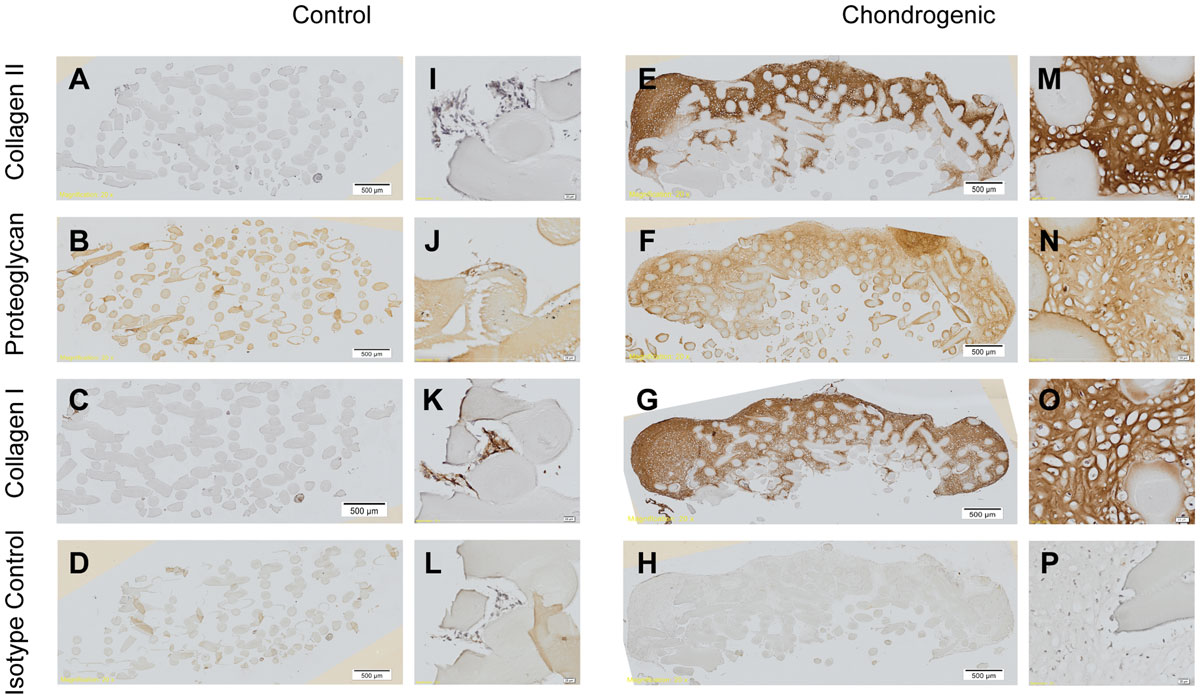
The Immunohistochemistry, the process by which scientists detect the composition of a cellular tissue, demonstrated positive staining for collagen type II and cartilage proteoglycans, as well as collagen type I, which are all components of the extracellular matrix in cartilage.
Real time PCR analysis demonstrated up-regulation of collagen type II, aggrecan, and SOX9 genes when IPFP-ASCs were stimulated by TGFβ3 and BMP6. Thus, IPFP-ASCs can successfully undergo chondrogenesis using TGFβ3 and BMP6 and the cartilage-like tissue that forms on the surface of 3D-printed chitosan scaffold may prove useful as an osteochondral graft.
This research is different from previous works involving IPFP-ASCs induced chondrogenesis because the new cartilage cells did not actually penetrate the scaffold, as much as they formed a “cap” on top of it. “We have seen this phenomenon in micromass cultures of these cells without scaffold,” the researchers wrote, adding that they believe “this more accurately mimics the behavior of the formation of an osteochondral unit whereby chondrocytes are fully surrounded in their own matrix supported at their base by subchondral bone which provides strength and support.”
The construct may thus provide the basis for viable cartilage repair and in-vivo implantation. Either way it seems safe to say that this is yet another sign that full cartilage regeneration will soon be within the reach of our medical capabilities.



