Researchers from the University of Minnesota have developed a novel bio-ink, enabling them to create a functional 3D printed beating human heart.
The cell-laden biomaterial, produced using pluripotent stem cells, allowed the research team to 3D print an aortic replica with more chambers, ventricles and a higher cell wall thickness than was previously possible. In future, organs replicated utilizing this process, could provide a test bed for a variety of drugs and devices, as well as a model for genetic diseases.
“This approach could be applied to many other cell types with poor proliferative and migratory capacity following differentiation,” stated the research team. “The living human pump shown here and future design iterations, will find utility for multiscale in vitro cardiology assays, injury and disease modeling, medical device testing, and regenerative medicine research that should more easily transfer to clinically relevant outcomes.”
Additive’s vascular applications
Early efforts at replicating cardiac tissues consisted of geometrically simple structures, made by casting cardiomyocytes in an ECM-based gel. While these tissues could be attached to posts, allowing them to contract and to modulate mechanical loading, their lack of complexity led to limited use within in vitro applications. In addition, such tissues could generate force, but weren’t able to pump fluid, which further restricted their ability to mimic vascular structures.
Attempting to address this issue, researchers have recently developed tissue models that are capable of replicating the pressure-volume dynamics of the heart. Nonetheless, these are often single-ventricle solutions, which aren’t capable of perfusion, a critical trait of genuine blood vessels and organs. Moreover, to enable casting of an ECM gel or seeding of cardiomyocytes after fabrication, many existing single ventricle models rely on a simple cup-like design. “These methods are suitable for an open, single-chamber structure that is wider on top than at the base; however, to generate an enclosed, perfusable model, more advanced fabrication technologies are required,” said the researchers.
Additionally, an increasing number of studies have demonstrated the ability to print entire heart organ models using biological materials, but these constructs have either lacked the cells or electromechanical functions to replicate the genuine article. This is due to the challenges associated with handling mature cardiac muscle cells, which do not proliferate or migrate readily, preventing researchers from achieving the high cell density required.
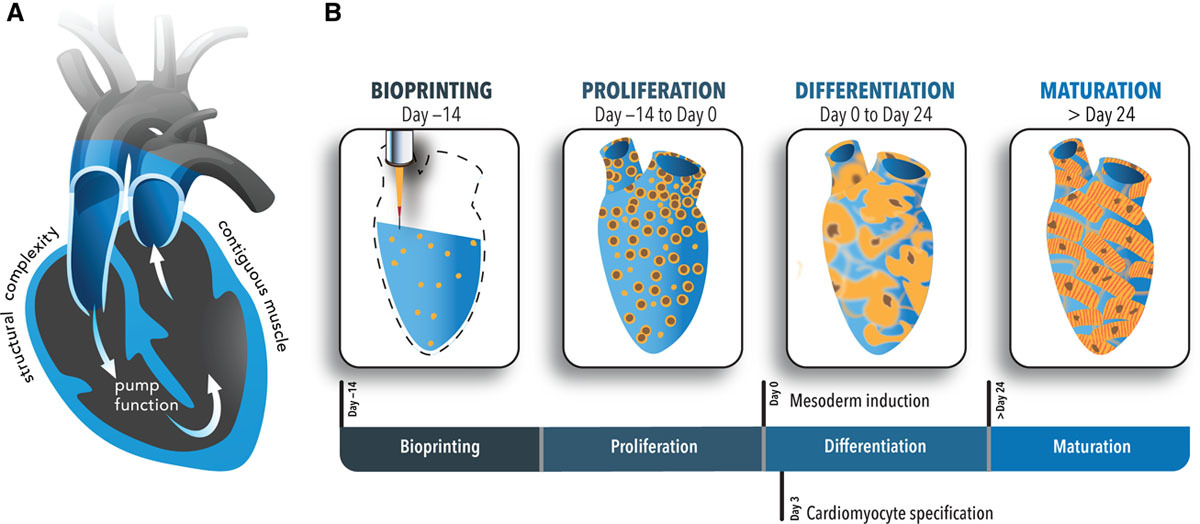
“Inclusion of contiguous, living muscle to support pump function has not yet been achieved. This is largely due to the challenge of attaining high densities of cardiomyocytes—a notoriously nonproliferative cell type,” explained the research team. “An alternative strategy is to print with human induced pluripotent stem cells, which can proliferate to high densities and fill tissue spaces, and subsequently differentiate them into cardiomyocytes in situ.”
As opposed to traditional casting methods, 3D bioprinting offers an accessible way for researchers to produce more complicated tissues from the ground up. Using highly proliferative stem cells, the Minnesota team aimed to induce the differentiation of cardiomyocytes in situ, customizing the tissues for cardiac applications.
For this approach to succeed, the team would need to develop a bio-ink formulation that promoted cell viability and enabled hiPSC proliferation and subsequent differentiation into cardiomyocytes. The researchers achieved this by tailoring an ECM formula from previous studies, to promote cardiomyocyte differentiation, and created a novel bio-ink that could be deposited with spatial fidelity.
3D printing the human heart
The process of creating the new bio-ink began with a gelatin methacrylate (GelMA) base material, which was later cross-linked via photoactivation to make the bio-ink printable. Further adding the chemicals fibronectin and laminin-111, as well as stem cell and ECM proteins, was found to support cardiomyocyte differentiation, and structural integrity. This formulation achieved a cell density of 0.1 mg DNA/g of gel, which is on the same order of magnitude as native cardiac tissue, making it ideal for experimentation.
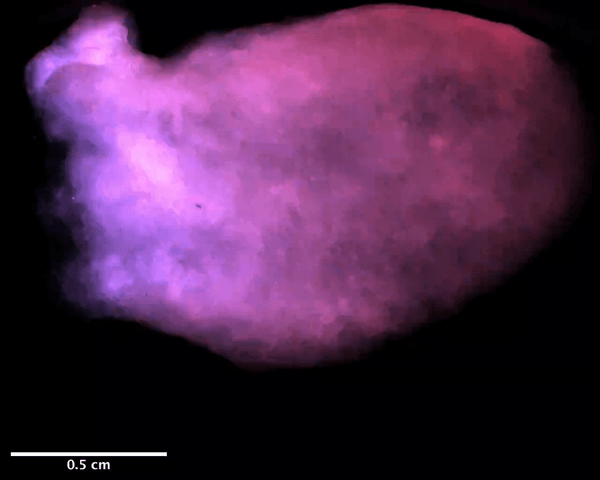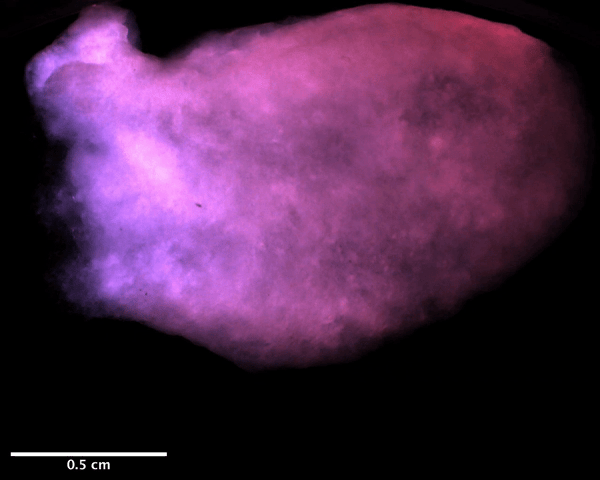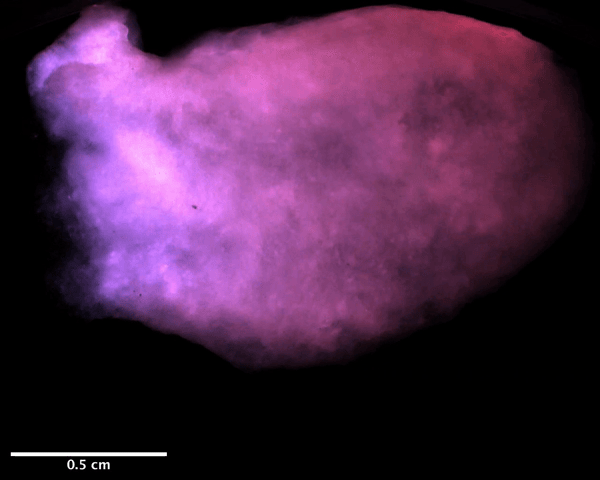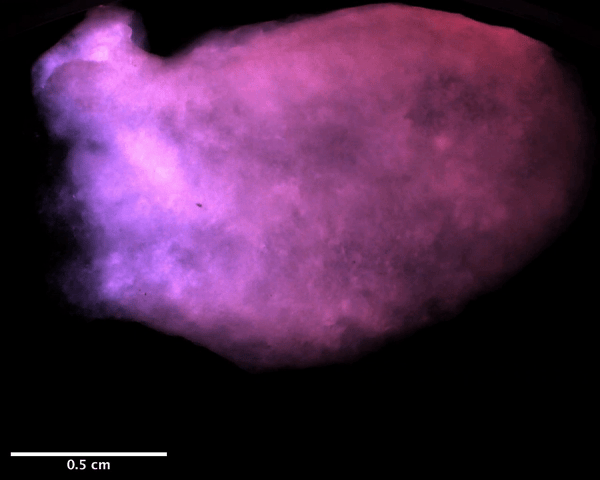
Using a magnetic resonance imaging scan of a human heart and the newly devised bio-ink, the team 3D printed stem cell–laden structures with two chambers and a vessel inlet and outlet. After the cells had multiplied to a sufficient density, the team differentiated the cells within the structure, giving them different roles within the heart. While the septum between the ventricles was partially removed to provide entry for nutrient delivery, and the structure was limited to two vascular connections, the heart’s performance proved resilient and functional.
Testing the accuracy of their creation, the research team used magnetic resonance imaging scans to compare their aortic structure with a 3D digital reconstruction. Not only was 86 percent of the printed structure found to be within 0.5 mm of the template, but a cross-sectional comparison revealed a high level of fidelity within its interior chambers. After 14 days, 90 percent of the bio-ink volume was populated with cells from both singular and large colonies, and by six weeks, very limited cell death was observed, suggesting ongoing cell health in the replica’s aortic structure.
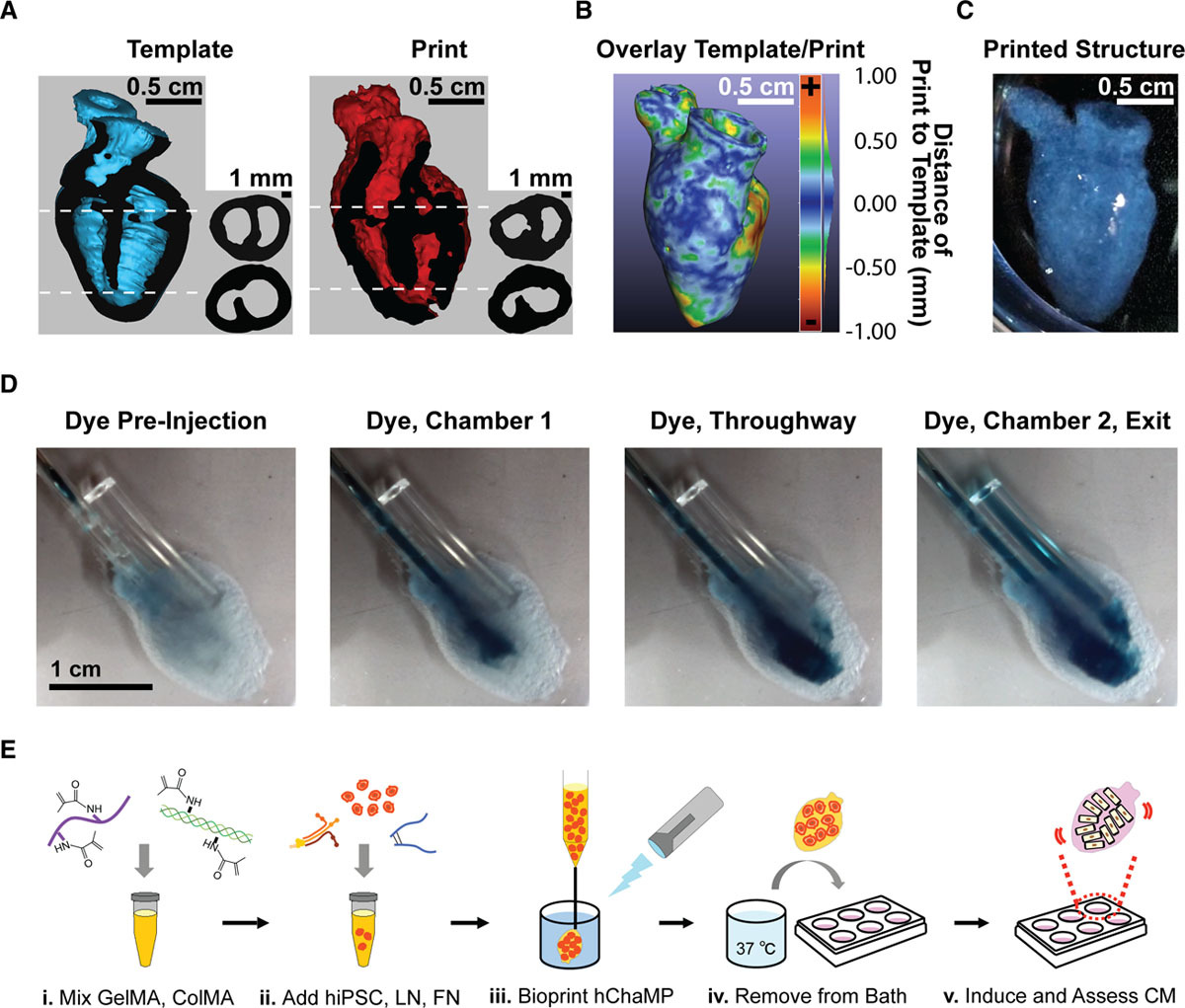
Moreover, the routine fabrication of the heart cells meant that it was capable of pumping blood or ‘beating.’ In order to determine the extent to which electromechanical function was preserved throughout the vessel, electrical function was measured via calcium transients of randomly selected regions over six weeks. Peak amplitude did not change over that time, nor did beating frequency, and the artificial organ showed the potential for lasting considerably longer than six weeks. Optical mapping was then used to measure voltage changes throughout the entire structure. The resulting action potentials detected on the heart’s surface, reflected the researchers’ predicted response to altered pacing frequency and drug stimulation.
As a result, the Minnesota team concluded that they had successfully created the first robust electromechanical function in a perfusable, chambered cardiac pump. The heart demonstrated the same behaviours after six weeks, and at its densest regions, the organ’s wall thickness exceeded 500 μm. Not only is this greater than that displayed by single ventricle models, but the team found that higher cell layer thickness would increase the volume output of the replica heart, resulting in a more robust pump function. The resulting macroscale human heart structure is able to be examined on multiple scales, offering plenty of potential pharmaceutical benefits in future. This could greatly support medical device testing and preclinical cardiology, and push research closer to clinical transplantation.
“This advance represents a critical step toward generating macroscale tissues, akin to aggregate-based organoids, but with the critical advantage of harboring geometric structures essential to the pump function of cardiac muscle,” concluded the research team. “Looking forward, human chambered organoids of this type might also serve as a test bed for cardiac medical devices and eventually lead to therapeutic tissue grafting.”
Hearts produced using additive manufacturing
3D bioprinting has been used to create a range of vascular structures in recent years, often for testing and research purposes rather than transplantation. Biomedical researchers from Texas Tech University Health Sciences Center El Paso (TTUHSC El Paso) and The University of Texas at El Paso (UTEP) for instance, have developed mini-hearts using 3D bioprinting. These heart-tissue structures were sent to the International Space Station (ISS) to gain an insight into how microgravity affects the function of the human heart.
A team of scientists at Tel Aviv University (TAU), Israel, has also successfully engineered and printed an entire heart, “complete with cells, blood vessels, ventricles and chambers.” The research aimed to set a precedent for future work by exploring the possibility of creating highly-detailed and patient-specific, 3D printed tissues.
BIOLIFE4D meanwhile, a biotechnology company from Chicago, has also successfully demonstrated the ability to 3D print human heart tissue. The company aims to develop technology capable of 3D printing valves, blood vessels, miniature organs and a full human heart.
The researchers’ findings are detailed in their paper titled “3D printing the next generation of enhanced solid oxide fuel and electrolysis cells” published in the Circulation Research journal. The report was co-authored by Molly E. Kupfer, Wei-Han Lin, Vasanth Ravikumar, Kaiyan Qiu, Lu Wang, Ling Gao, Didarul B. Bhuiyan, Megan Lenz, Jeffrey Ai, Ryan R. Mahutga, DeWayne Townsend, Jianyi Zhang, Michael C. McAlpine, Elena G. Tolkacheva and Brenda M. Ogle.
You can now nominate for the 2020 3D Printing Industry Awards. Cast your vote to help decide this year’s winners.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows a diagram of the 3D printed heart produced by the Minnesota team. Image via the Circulation Research journal.



