Scientists at UMC Utrecht have made a number of innovations in volumetric bioprinting.
Bioprinting, the 3D printing of living cells and tissues, is an additive manufacturing technique offering potential for medical applications. However, 3D printing living cells and tissue continues to pose a number of challenges.
The UMC Utrecht researchers have made three key developments in this field: creating biologically functional regions in 3D printed cells; using granular gels to optimize 3D bioprinted cells; and combining volumetric bioprinting with melt electrowriting to 3D print blood vessels.
It is hoped that these developments will help to expand clinical use of 3D bioprinting.
3D printing cells with biologically functional regions
One key innovation relates to the biological functionality of 3D printed cells.
Volumetric bioprinting, used in conjunction with specialized gels, allows cells to be 3D printed in a matter of seconds. However, conventional 3D bioprinting methods don’t allow these cells to be accurately manipulated and placed exactly where they are needed. Moreover, the gels cannot be edited to enable the development, growth and specialization of cells.
The researchers worked to enable chemical changes to the 3D printed materials after the initial 3D bioprinting process. To achieve this, the researchers edited both the porosity of the gel, and the internal compounds which bind with other molecules in the gel.
“First we printed our gelatin-based constructs with the volumetric printer, then by infusing these constructs with biomolecules and photoinitiator, we could create complex 3D motives inside the gelatin structures,” explained Marc Falandt, an author of the Advanced Materials Technologies paper.
This makes it possible to volumetrically 3D bioprint tissue that can have growth factors or bioactive proteins “painted” into them.
Falandt sees this as a major step in creating smart materials that can be biochemically edited. “This approach is extremely promising for the creation of biofabricated scaffolds that could guide cell behavior and development.”
Using granular gels to 3D bioprint optimized cells
3D printed cells require special attention to ensure that they can survive and thrive. Moreover, it is vital that the cells can grow, move, and communicate.
Extrusion 3D printing allows for a variety of cell types to be deposited at high quantities. However, this process is time-consuming, gravity dependent, and can cause mechanical stress to the cells.
Whilst volumetric bioprinting does not possess the drawbacks in speed or gravity dependence, it does distribute the cells randomly, and in low numbers. Moreover, the cells cannot function and communicate effectively.
Therefore, materials such as soft hydrogels must be used, as these allow for the self-organization and communication of cells. Yet, traditional soft hydrogels pose problems when it comes to 3D print resolution and shape fidelity.
The scientists employed granulated resins to overcome these challenges.
“Granular gels are basically gel microparticles packed tightly together,” explained Davide Ribezzi, first author of a study posted to bioRxiv. “Packed microgel particles can be designed and customized to display a broad array of added useful properties.”
During extrusion 3D printing, cells and other chemicals can be quickly and accurately deposited in the resin. Volumetric 3D printing is then used to complete the process by creating the shapes around the extruded cells.
Experimentation with cells highlighted that granulated resins enable more biological activity after printing. Within eight days of being 3D printed, stem cells were able to spread out more, epithelial cells created more junctions, and neuron-like cells made more connections.
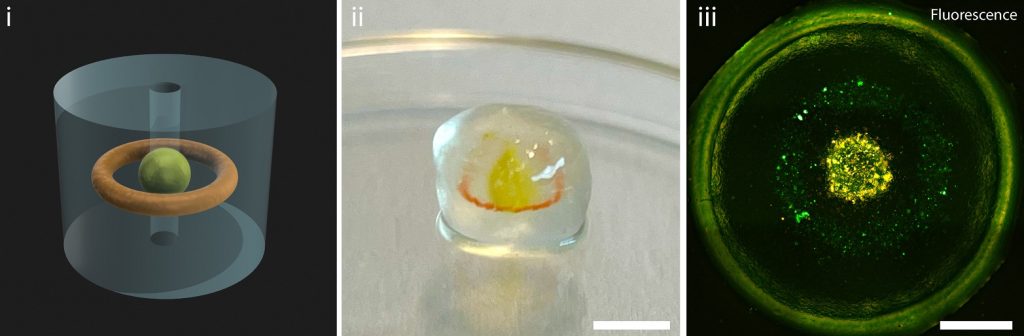
Merging bioprinting techniques for stronger, functional blood vessels
Because volumetric bioprinting uses cell-friendly gels, the final 3D printed structures are often weak. This poses problems when producing blood vessels, which need to withstand high pressure and bending. Therefore, the researchers combined volumetric bioprinting with melt electrowriting to create stronger and more durable structures.
Melt electrowriting uses a narrow filament of molten plastic to 3D print intricate and strong scaffolds. However, due to the high temperatures involved, electrowriting cannot directly 3D print cells.
Thus, volumetric bioprinting was incorporated to solidify cell-laden gels onto the scaffolds. The 3D printed tubular scaffold is submerged into a vial of photoactive gel, and then placed in the volumetric printer. The laser of the 3D printer can then selectively solidify the gel onto the scaffold.
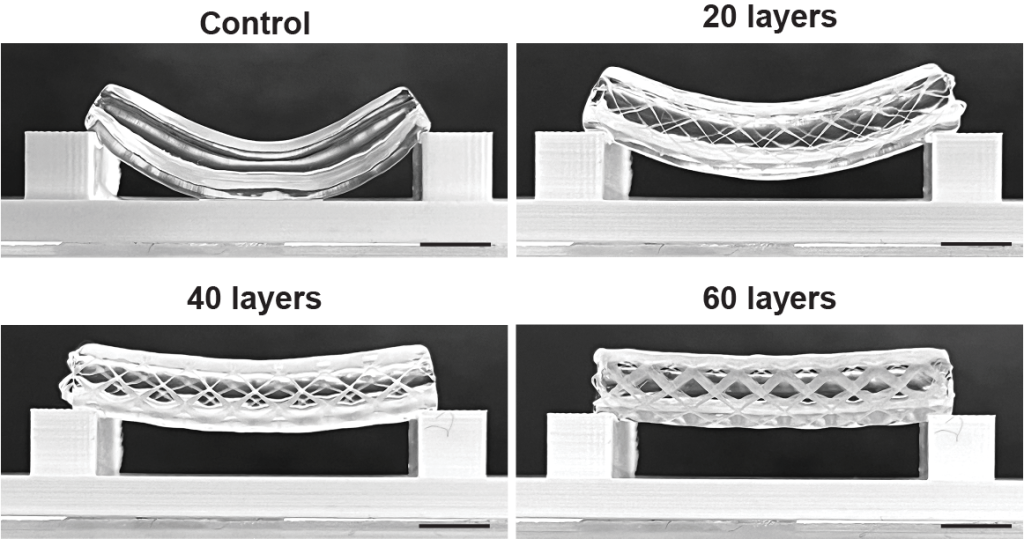
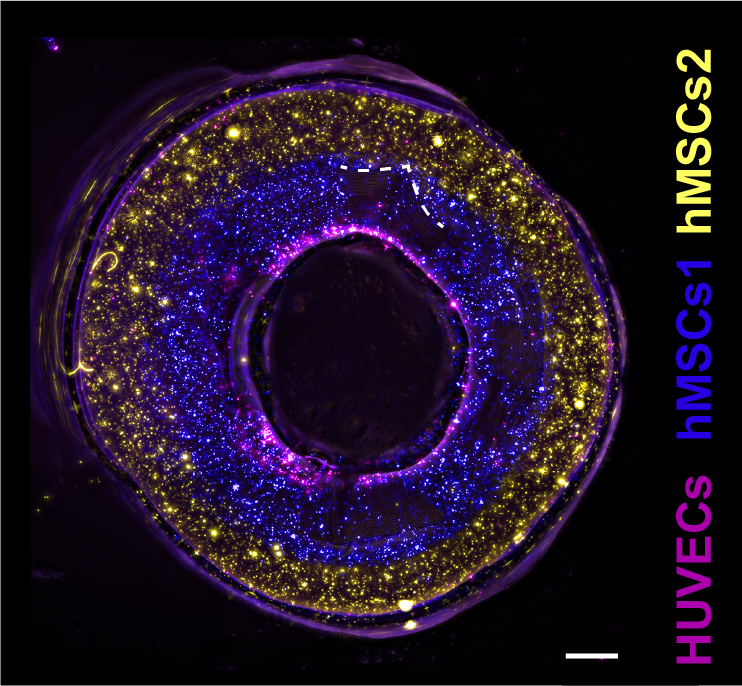
In a study published in the Advanced Materials journal, researchers tested various scaffold thicknesses, and different placements of the bioprinted gels. The research team also 3D printed a proof of principle blood vessel with two layers of stem cells, and a layer of epithelial cells in the center.
This design allows holes to be included in the side of the 3D print, providing the possibility for controlled permeability of the vessel. More complex structures were also produced, including forked vessels, and vessels with venous valves.
“This was a proof of principle study. What we now need to do is replace the stem cells with functional cells that are part of a real blood vessel,” commented Gabriël Größbacher, lead author of the study.
Developments in 3D bioprinting
Earlier this year 3D Systems announced plans for a Regenerative Tissue Program (RPT) to develop and commercialize bioprinted human tissue. The first RPT to be developed under the RPT is patient-specific regenerative breast tissue (RBT).
Using 3D modeling and 3D bioprinting alongside a Virtual Surgical Planning (VSP) system, 3D Systems can reportedly design and 3D print bio-integrative scaffolds that match the anatomy and physiology of the patient.
Elsewhere, researchers at the University of Swansea are developing a 3D printed vegan nose, for those requiring artificial nose transplants. In this process, nanocellulose hydrogel and hyaluronic acid are used as bioink to 3D print an artificial cartilage scaffold. This is then cured by a biological catalyst to increase its strength. The 3D printed scaffold is then bathed in a solution of the patients’ cartilage cells, before being surgically implanted.
Subscribe to the 3D Printing Industry newsletter to ensure you keep up with the latest 3D printing news. You can also follow us on Twitter, like our Facebook page, and subscribe to the 3D Printing Industry Youtube channel to access more exclusive content.
Are you interested in working in the additive manufacturing industry? Visit 3D Printing Jobs to view a selection of available roles and kickstart your career.
Featured image shows the embedded printing of cells in a granular gel. Photo via LevatoLab, UMC Utrecht.


