Materialise is the first company to receive FDA clearance for software intended for 3D printing anatomical models for diagnostic use, including its Materialise Mimics Innovation Suite.
The news follows an August 2017 announcement by the FDA stating that software for use in creating 3D printed patient-specific anatomical models would be regulated as class II medical devices.
3D printed anatomical models in surgical planning
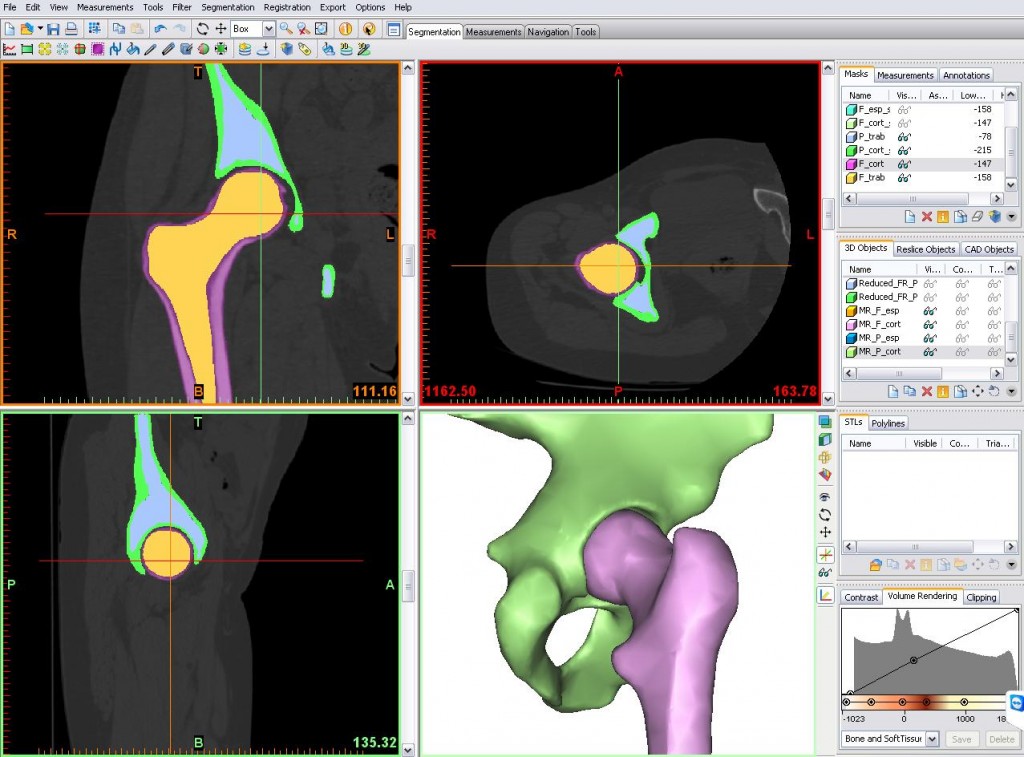
Materialise’s Mimics is a medical imaging tool used for the analysis and creation of accurate 3D printable anatomical models. Hospitals are turning to 3D visualisation tools such as Mimics and 3D printing to improve surgery times and costs.
Patient-specific 3D printed models allow physicians to better practise and visualise surgical operations, compared to simply viewing a 3D model on a computer monitor. Rady Children’s Hospital, with a team from the University of California, have found 3D printed anatomical models can help reduce surgery times by approximately 25 percent.
FDA ensures safe development of medical 3D printing applications
Top US hospitals have already realised the benefits of 3D printed anatomical models. 16 of the top 20 US hospitals, ranked by the US News and World Report, have implemented 3D printing using Materialise’s Mimics software.
In December 2017 Todd Pietila, Materialise’s Business Development Manager for Hospital 3D Printing, spoke to 3D Printing Industry about the new FDA guidance for manufacturers of 3D printed devices.
Pietila praised the FDA’s “efforts to provide advice based on in-depth research on Additive Manufacturing for medical applications. We believe in the FDA’s commitment to supporting the development of 3D Printing and the promises it holds, ensuring safe and effective implementation of 3D printing applications in hospitals.”
Dr Frank J. Rybicki, Chief of Medical Imaging at Ottawa Hospital said “510k clearance is an essential component to ensure quality and safety in the practice of anatomical modeling in hospitals. This milestone for Materialise serves as a benchmark for the clinical implementation of 3D printing for physicians creating 3D models at the point-of-care.”
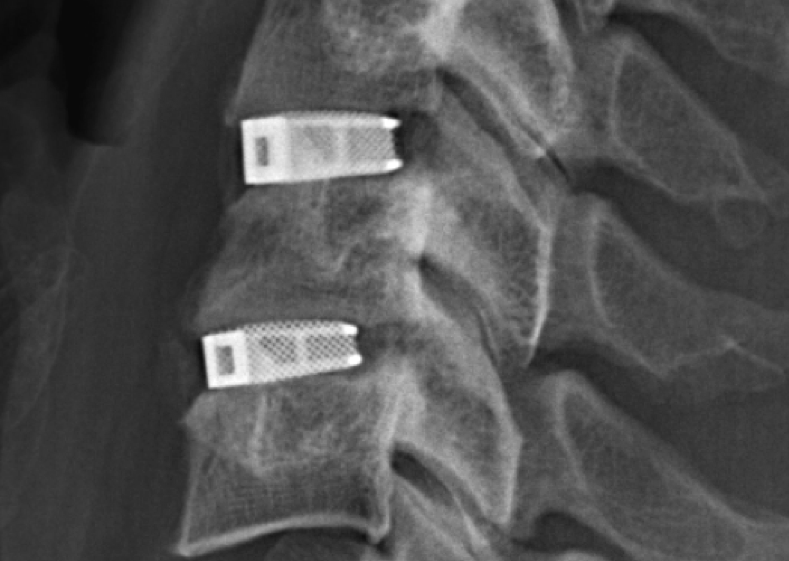
In January the FDA cleared the first 3D printed spine implant, for use in spinal injuries and defects. The implant by Emerging Implant Technologies GmbH uses SLM 3D printing to produce implants with a core that mimics structure of bone. Carbon has recently received FDA approval for their 3D printable DENTCA resins.
Keep up to date with the latest medical applications of 3D printing. Subscribe to the 3D Printing Industry newsletter, follow us on Twitter and like us on Facebook.
Vote for your the 3D printing medical, dental or healthcare application of the year in the 2018 3D Printing Industry Awards.
Our 3D Printing Jobs service is now live. Post a job or advance your career in 3D printing now.
Protolabs is sponsoring the 2018 3D Printing Industry Awards design competition. Enter now for the chance to win a 3D printer.
Featured image shows CT images viewed in Materialise’s Mimics software. Image via Materialise.


