Germany-based RWTH Aachen University’s Digital Additive Production facility (DAP) is researching a new zinc-magnesium alloy combination for lattice structures as part of the BioStruct project. DAP says Laser Beam Powder Bed Fusion (PBF-LB) is the only method capable of producing these structures.
Current bone defect treatments, like titanium implants and autologous bone grafts, have limitations in treating critical-size bone defects. This weakens surrounding bone tissue, not making them ideal for large defects. To tackle these drawbacks, the BioStruct consortium is working on a bioresorbable implant concept allowing patient-friendly bone healing. The challenge is to choose suitable materials and geometries for the body in addition to PBF-LB processing. Zinc and magnesium alloys have shown “promising” in the development of resorbable bone implants, says DAP.
Laser Beam Powder Bed Fusion – a new hope for patient-specific implants?
Laser Beam Powder Bed Fusion opens new design options for implants to meet patient-specific needs, such as mechanical stress and corrosion behavior at the application site. An algorithmic lattice structure design approach is used, in which the geometry and arrangement of individual struts or lattice cells are created parametrically according to the specified requirements. The produced lattice structure is tailored to the location of the bone defect and prepared for production with PBF-LB.
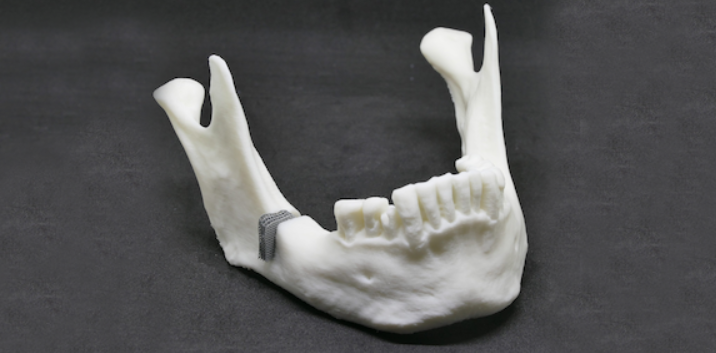
The scientists accomplished grain refinement and targeted microstructure adjustment in their research by adding small quantities of magnesium to zinc. This ZnMg alloy was used to effectively and reproducibly manufacture the first demonstrator as a lattice-structured jawbone implant. The lattice structure used in the demonstrator has a strut diameter of 200 μm.
The BioStruct project’s findings will be further expanded in the reACT alliance. Ready-to-use demonstrator implants will be created based on the knowledge acquired from the production and biocompatibility of the ZnMg demonstrator implants. Additionally, the design process will also be optimized and automated.
Members of the DAP chair are creating a material and post-processing-specific database, along with an application-specific database, to automatically integrate patient and production-related needs into the design process. The reACT alliance’s sub-project is completed with the prototypical realization of a novel design and material concept and the exploration of design assistants. The coalition’s overarching objective is to produce customized, bioresorbable implants that cater to patient-specific requirements and allow for gentler therapy.
Advancements in bone implants via additive manufacturing
Engineers from the Delft University of Technology utilized extrusion-based 3D printing to produce temporary bone implants with porous iron. These biodegradable implants made of porous iron have significant potential as a substitute for bone in the short term, as they break down as new bone develops in their place. By being absorbed by the body, temporary implants can help mitigate the risk of prolonged inflammation, which is often a problem with permanent metal bone implants such as those made of titanium. Amir A. Zadpoor, the lead author of the study, said, “In comparison with other biodegradable metals or polymers for bone implants, iron has a high mechanical strength, which allows for the design and fabrication of porous structures for the treatment of critical bone defects.”
University of New South Wales (UNSW) researchers created a novel technique for 3D printing bone-mimicking structures comprising living cells, with potential applications in bone tissue engineering, disease modeling, and drug screening. Associate Professor Kristopher Kilian says the method could facilitate in-situ reconstruction of cartilage and bone defects by extruding the ceramic-based ink directly into the affected region. Kilian worked with Dr. Iman Roohani of UNSW’s School of Chemistry on the discovery, which enabled the printing of cell-laden “bone” at room temperature for the first time.
What does the future of 3D printing for the next ten years hold?
What engineering challenges will need to be tackled in the additive manufacturing sector in the coming decade?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows a Mandibular model made of PLA with a defect-fitting implant made of ZnMg additively manufactured based on RWTH DAP’s newly developed design and alloy concept. Image via RWTH Aachen University.



