US 3D printer OEM Carbon has announced the development of an experimental bioresorbable material with demonstrable biocompatibility in vivo.
During testing, the in-house developed elastomer is said to have been designated ‘non-toxic,’ while being customizable to ensure that it’s fully absorbed into host tissues. As such, Carbon says the resin could be ideal for 3D printing lattices with applications in tissue repair, wound dressing or the creation of nerve conduits.
“We’re very pleased to announce that Carbon’s developmental bioabsorbable elastomer platform has demonstrated biocompatibility in vivo,” said Jason Rolland, SVP of Materials at Carbon. “These intricate structures made with Carbon Digital Light Synthesis (DLS) technology may hold the key to addressing the longstanding challenge of optimizing the mechanical properties and degradation rate of an implant.”
“It’s a milestone, and we look forward to working with interested partners to further develop applications for this resin.”
Digital Light Synthesis technology
Based in Redwood City, California, Carbon’s 3D printing portfolio revolves around its proprietary DLS technology. In essence, the process involves using digital light projections and oxygen-permeable optics to cure photopolymer resins into 3D parts. Alongside DLS-equipped 3D printers, which are marketed via a subscription model, the firm also offers a range of engineering-grade materials.
This product lineup now includes resins that vary from the rigid, fatigue-resistant FPU 50, to the dental-focused DPR 10, with each featuring characteristics that make them attractive within certain use cases. EPU 40, for instance, offers high impact absorption, making it ideal for creating vibration-prone parts like gaskets, while CE 221 is said to be great for producing fluid routing parts with high thermal stability.
When it comes to medical materials, Carbon has previously dipped its toe in the water with resins like MPU 100, which is biocompatible and sterilizable. However, the company’s offering lacks an elastomer that’s bioresorbable i.e. that can be absorbed into the human body, a trait that’s increasingly in demand from medical implant manufacturers, and it now aims to change this via its elastomeric R&D.

Carbon’s experimental elastomer
As it’s still under development, Carbon hasn’t yet named its experimental elastomer, but if the results of its latest testing are anything to go by, it could have significant potential in the healthcare sector. According to the firm, its latest milestone study showed that the material offers “impressive mechanical performance, biocompatibility and tunability.”
This could be particularly important when it comes to tailoring the resin’s absorption rate to meet different clinical applications. With the elastomer having also shown the required tissue tolerance and desirable healing responses for an implantable device through 26 weeks, Carbon now believes it has the potential to address a whole host of use cases.
When dressing wounds or repairing tissues like tendons, biomedical grafts are often used to reduce inflammation and accelerate healing. Given its resin’s enhanced elasticity and directionality, as well as its consistency of mechanical properties, Carbon believes it can address such applications in a way that limits any need for repeat operations, and allows patients to move more freely once treated.
In the production of artificial nerve conduits, the company also sees its material as having the qualities needed to provide enhanced flexibility, porosity, neuro inductivity and neuro conductivity. Post-surgery, meanwhile, the firm says its elastomer can be used to create absorbable lattice ‘cushions,’ which prevent bleeding or leakage from occurring in soft tissues as they move during healing.

Bioresorbable 3D printing advances
With 3D printed medical implants beginning to gain traction in certain parts of the healthcare industry, an increasing amount of R&D is being committed to making these bioresorbable. BellaSeno’s 3D printed breast scaffolds, which are designed for implantation during breast regeneration surgery, were used in human trials for the first time this year.
Similarly, Particle3D has patented a bioresorbable implant 3D printing material. Composed of ceramic suspended in a fatty acid matrix, the firm’s biomaterial enables the production of patient-specific grafts with ‘bone-like’ porosity.
Elsewhere, larger bioprinting firm BICO has begun working with start-up Nanochon to develop 3D printed regenerative joint implants. Nanochon says that once ready, the implants will deliver faster and more successful recovery rates for patients, while reducing the cost of treatment for healthcare providers.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? featuring discussion, debriefs, video shorts and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
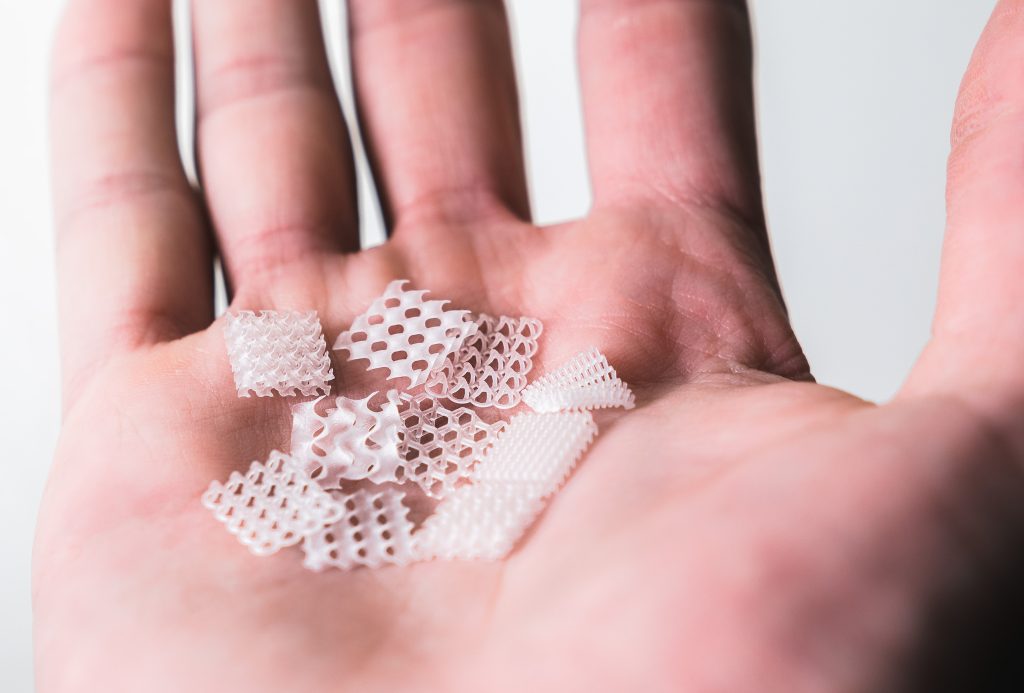
Featured image shows a set of demonstrator parts 3D printed from Carbon’s developmental elastomer. Photo via Carbon.


