Health Canada, the national public health department of the Government of Canada, has released a draft guidance document for manufacturers intending to license implantable 3D printed medical devices.
The document states, “Due to the fast-changing technological environment, Health Canada will continue to adapt its policy approach to 3D-printing as issues on the topic evolve,”
“This guidance document therefore represents the first phase of 3D printing policy in Canada.”
Licensing Requirements for 3D printing medical devices
The draft guidance document remains consistent with the standard definitions from the International Medical Device Regulators Forum (IMDRF). The document states, “hospitals that manufacture 3D printed implantable medical devices for distribution beyond their institution qualify as a manufacturer, and should comply with the same obligations as other medical device manufacturers.”
As a result, such manufacturers are instructed to consider the design philosophy, biocompatibility, performance, market history, and packaging of a 3D printed medical device.
“If the application is to modify a licensed device, a description of the modification is required. Examples of device modifications related to 3D printing may include, but are not limited to [a] 3D printed component that is being added to an approved device (i.e., porous surface coating) [and] a 3D printed component that is to be used with an approved device (i.e., dental superstructures/abutments/implants).”

Safety requirements of 3D printed medical devices
Within the draft guidance document, safety considerations have also been proposed to ensure the effectiveness of 3D printed medical devices. This includes post-processing and sterilization, animal studies and clinical studies, software verification and validation, as well as shelf life studies for the device.
Furthermore, according to the device description policies, manufacturers must state whether 3D printing was used to produce only a part of the component or to produce the device in entirety. The draft guidance is currently seeking stakeholder feedback on the technical considerations outlined until 8 January, 2019.
3D printing for the healthcare sector
The use of additive manufacturing within the medical sector has seen great progress over the past few years. As a result, the U.S. Food and Drug Administration (FDA) Commissioner Dr. Scott Gottlieb released a statement reaffirming the agency’s commitment to a “new era of 3D printing of medical products.” This statement also revealed the FDA’s guidance for manufacturers submitting 3D printed devices for approval. On the announcment Dr. Gottlieb stated:
“3D printing is certain to alter the daily practice of medicine where patients will be treated with medical products manufactured specifically for them.”
Stay updated with the latest additive manufacturing news subscribe to the 3D Printing Industry newsletter. Also, find us on Facebook and like us on Twitter.
For new opportunities across additive manufacturing visit 3D Printing Jobs.
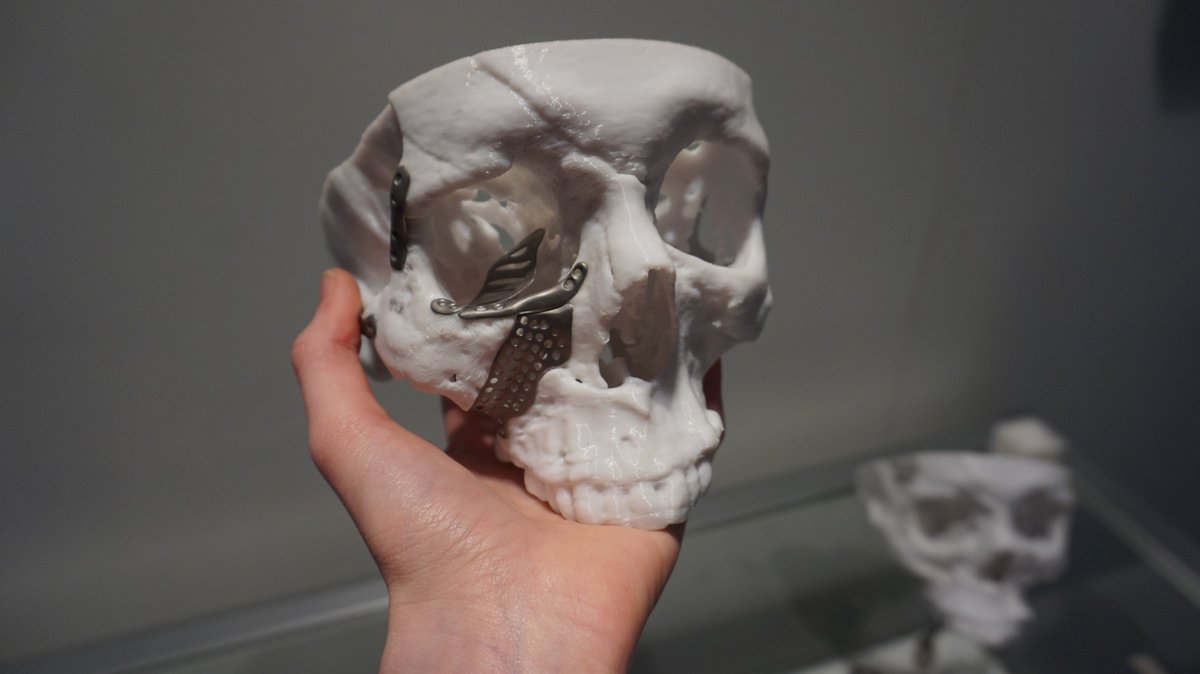
Featured image shows a 3D printed skull and implant by Renishaw. Photo by Beau Jackson.

