Desktop Health, the healthcare business unit of Massachusetts-based industrial 3D printer manufacturer Desktop Metal (DM) has announced a new formulation of its Flexcera resin for 3D printing gingiva.
Called Flexcera Base Ultra+, the 3D printable, light-curable resin is reportedly 50% stronger than its predecessors, with 70% greater resistance to deformation than ISO standards.
Desktop Health’s new material offering is optimized for the production of 3D printed gingiva components of full and partial removable dentures.
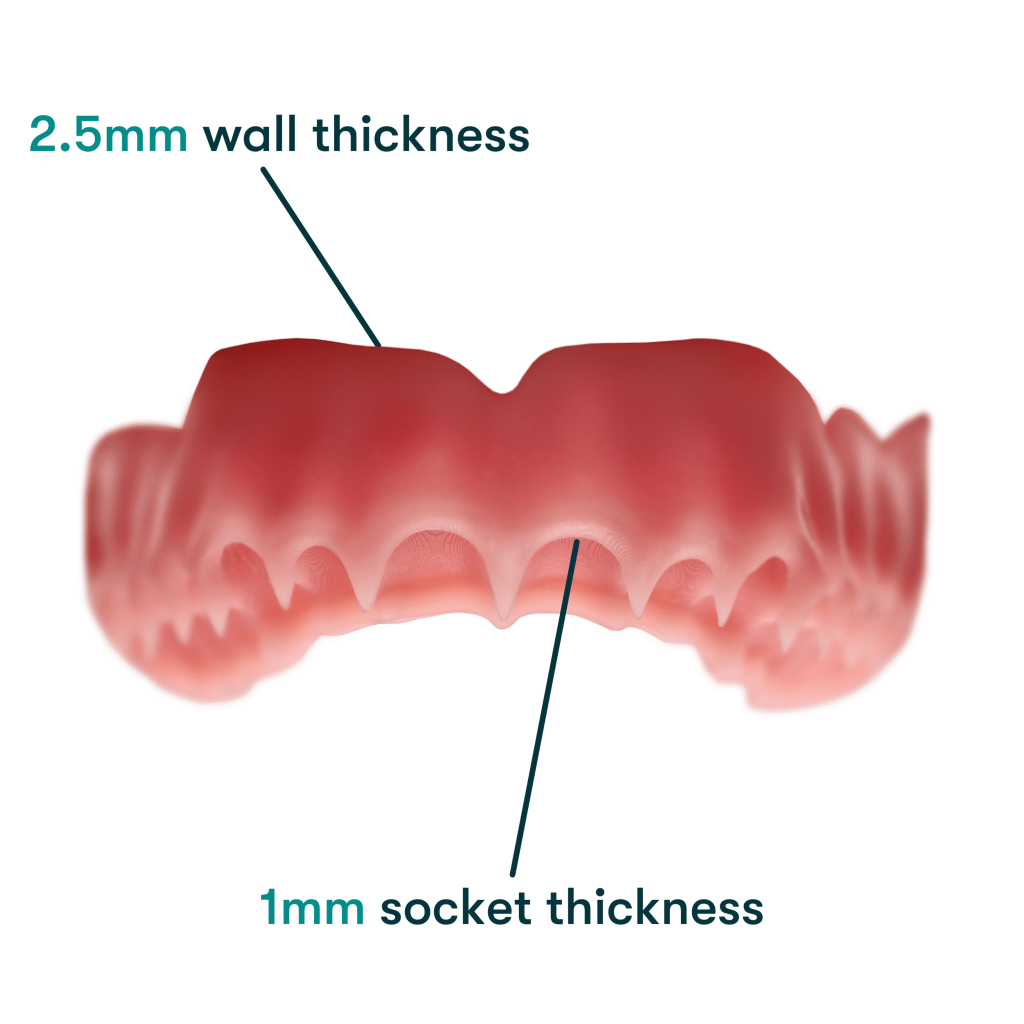
The new formulation is a nano-ceramic composite resin that enables the fabrication of thinner designs of 2.5 mm wall thickness and 1 mm socket thickness. This is said to allow for improved durability and a better fit, thanks to its improved flexural strength
Flexcera Base Ultra+ has received US Food and Drug Administration (FDA) 510(k) Class II approval, and is a CE Marked and MDR Class I certified medical device. The material is available in five natural gingiva shades, including a new pink coloring designed to closely resemble industry standards.
“New Flexcera Base Ultra+ demonstrates how we listen to customers and give them what they’re asking for, such as a firmer final product in an industry favorite shade,” commented Desktop Metal CEO Ric Fulop. “With more dental professionals than ever adopting a digital workflow and an aging population, the popular Flexcera family fits a growing need in the marketplace.”

Desktop Health 3D printing adds to growing dentures market
According to Desktop Health, the global dentures market was valued at $2.8 billion in 2023, and is expected to grow at a CAGR of 7.5% from 2024 to $4.6 billion in 2030.
The Flexcera resin portfolio includes a range of formulations designed to help dentists and dental labs efficiently deliver affordable 3D printed dentures for their edentulous patients. Back in 2021, Desktop Metal announced that it has received FDA approval for its first denture 3D printing resins, Flexcera Smile and Flexcera Base.
Lou Azzara, President of Desktop Health, claims that the newest addition to the firm’s resin portfolio 3D prints 26% faster than its predecessor when used with Einstein and Einstein Pro XL 3D printers. This is said to enable the production of 3D printed dentures at scale, without compromising on quality.
In testing, the Einstein Pro XL produced 21 Flexcera Base Ultra+ denture arches in 90 minutes, and reduced post-processing times for a more efficient workflow. The new material is also advertised as being 2X more resistant to moisture than Flexcera Smile, and is said to offer life-like luster and translucency .
The launch of Flexcera Base Ultra+ follows last year’s announcement that Desktop Metal defeated a shareholder lawsuit alleging that it lied about the FDA approval of its Flexcera dental resins to artificially inflate stock prices. The ruling found that DM’s disclosures with respect to its regulatory compliance requirements were not misleading.
3D printing enhances dental industry
When surveyed on the near-term 3D printing trends to watch out for in 2024, 3D printing experts highlighted additive manufacturing as playing a key role in improving personalization in the dental industry. In particular, the industry is expected to move from indirect production for mold creation to direct 3D printing of dental devices and appliances.
Austrian ceramic 3D printing company Lithoz is set to unveil the next development stage of its lithium disilicate material for dental 3D printing at LMT Lab Day Chicago 2024.The new material has been jointly developed integrated dental solutions manufacturer Ivoclar, being based on its IPS e.max lithium disilicate powder.
Lithoz claims that its new material will enable serial production of patient-specific, natural-looking all-ceramic dental restorations. The new lithium disilicate material can be used with its Lithography-based Ceramic Manufacturing (LCM) technology to 3D print up to 50 individual restorations in a single run.
Elsewhere, Microscale 3D printer manufacturer Boston Micro Fabrication launched UltraThineer, the “world’s thinnest cosmetic dental veneer.” Produced using projection micro stereolithography (PµSL), the 3D printed veneers are designed to be three times thinner than their conventionally manufactured counterparts. This is said to drastically reduce preparatory steps required by dental practitioners.
Subscribe to the 3D Printing Industry newsletter to keep up to date with the latest 3D printing news. You can also follow us on Twitter, like our Facebook page, and subscribe to the 3D Printing Industry Youtube channel to access more exclusive content.
Are you interested in working in the additive manufacturing industry? Visit 3D Printing Jobs to view a selection of available roles and kickstart your career.
Featured image shows Flexcera Base Ultra+ resin. Image via Desktop Health.


