Finland-based bioprinting firm Brinter has teamed up with Tampere University’s Kellomäki Lab Biomaterials and Tissue Engineering Group to discover new bioinks for 3D printing and advance progress within the bioprinting field.
The first breakthrough for the partners is the development of a new method for creating a photocrosslinkable bioink from gellan gum, a precursor proven to possess good rheological properties for hydrogels.
According to the team, their two-step crosslinking technique is an effective approach to turning unprintable gellan gum inks into feasible bioinks for the fabrication of 3D printed structures, and could also be applied to the formation of other bioinks.
Brinter’s 3D bioprinting activities
Brinter was founded in 2020 as a spin-out from 3D printing service bureau 3DTech Ltd, and specializes in developing 3D bioprinters, modules, and inks for clients in the biotech, pharmaceutical, research, and cosmetic industries. So far, the firm’s clients include the likes of Nanoform, Johannes Gutenberg University of Mainz, the University of Oulu, and Tampere University, the latter with whom Brinter is working on the development of new bioinks.
In June last year, the firm received €1.2 million in seed funding to expand its operations and take its bioprinting offering to the US and European medical markets.
The company’s flagship bioprinter is the Brinter One, a portable multi-material machine capable of printing both stiff and soft materials. In November, the firm launched its entry-level model, the Brinter Core, developed with the aim of making bioprinting more accessible. The printer is half the size and cost of its predecessor to enable more pharmaceutical firms, hospitals, universities, and research centers to access its 3D bioprinting technologies.
Most recently, Brinter partnered with extrusion technology developer Puredyne to develop and launch its new Visco Bio printhead for use with its bioprinters. The modular printhead is designed to extrude more precise and constant volume per revolution, meaning its customers can minimize their costly medical-grade material waste.

Developing photocrosslinkable bioinks
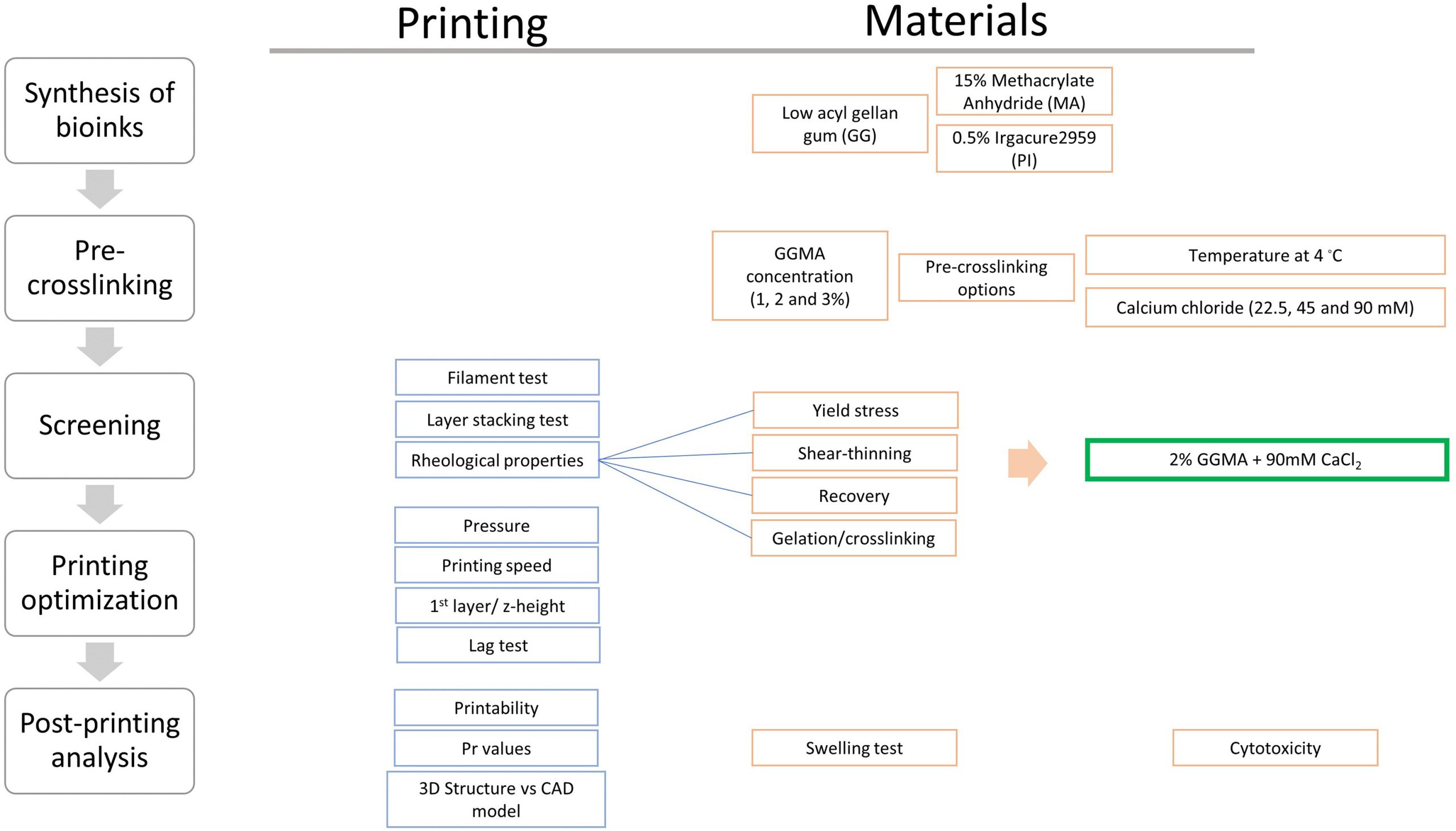
A lack of suitable bioinks has been identified as one of the major bottlenecks currently limiting progress within the field of bioprinting. The Brinter and Tampere University team sought to widen the scope of printable biomaterials with their latest study, which focuses on methacrylated gellan gum (GGMA).
Proven to exhibit good rheological properties, gellan gum is a precursor used as a thickening agent in food and cosmetic products, and in industrial settings. However, GGMA precursors alone are unable to maintain a stable filament shape after extrusion.
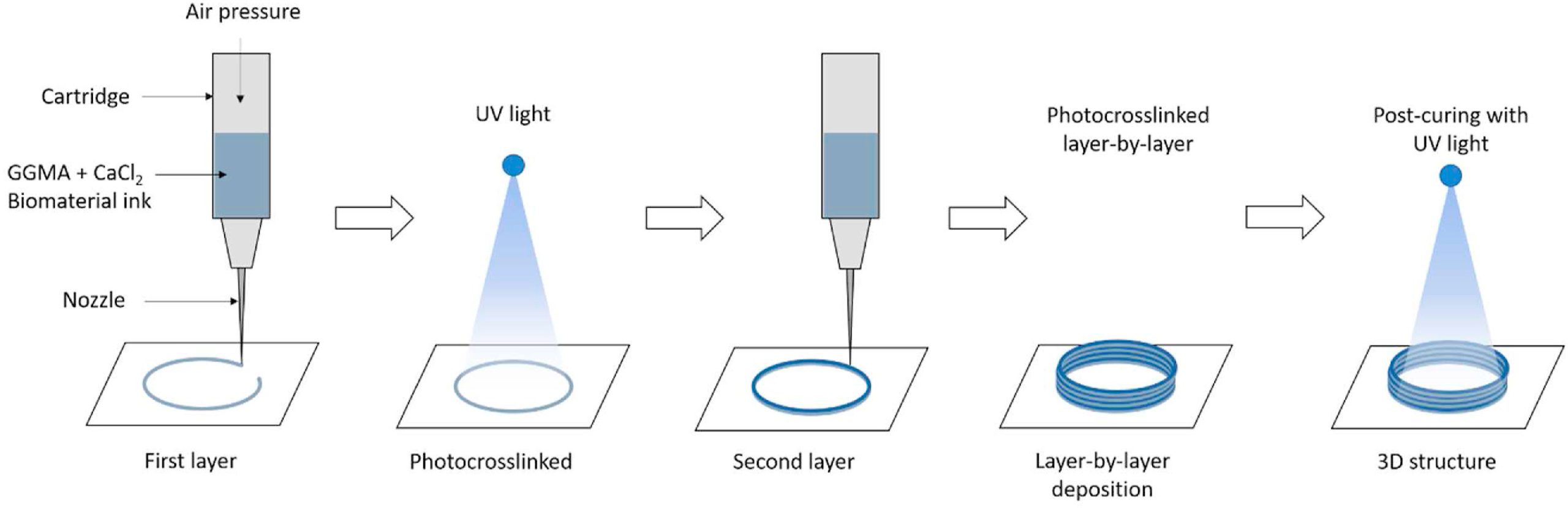
To overcome this and make GGMA suitable for printing, the team developed a new two-step photocrosslinking technique that made use of a Ca2+ ionic crosslinker. In the presence of the crosslinker, the GGMA precursor transformed from a liquid to a weak extrudable hydrogel. The team then used their photocrosslinking method to turn the weak hydrogel into a hydrogel with good shape fidelity.
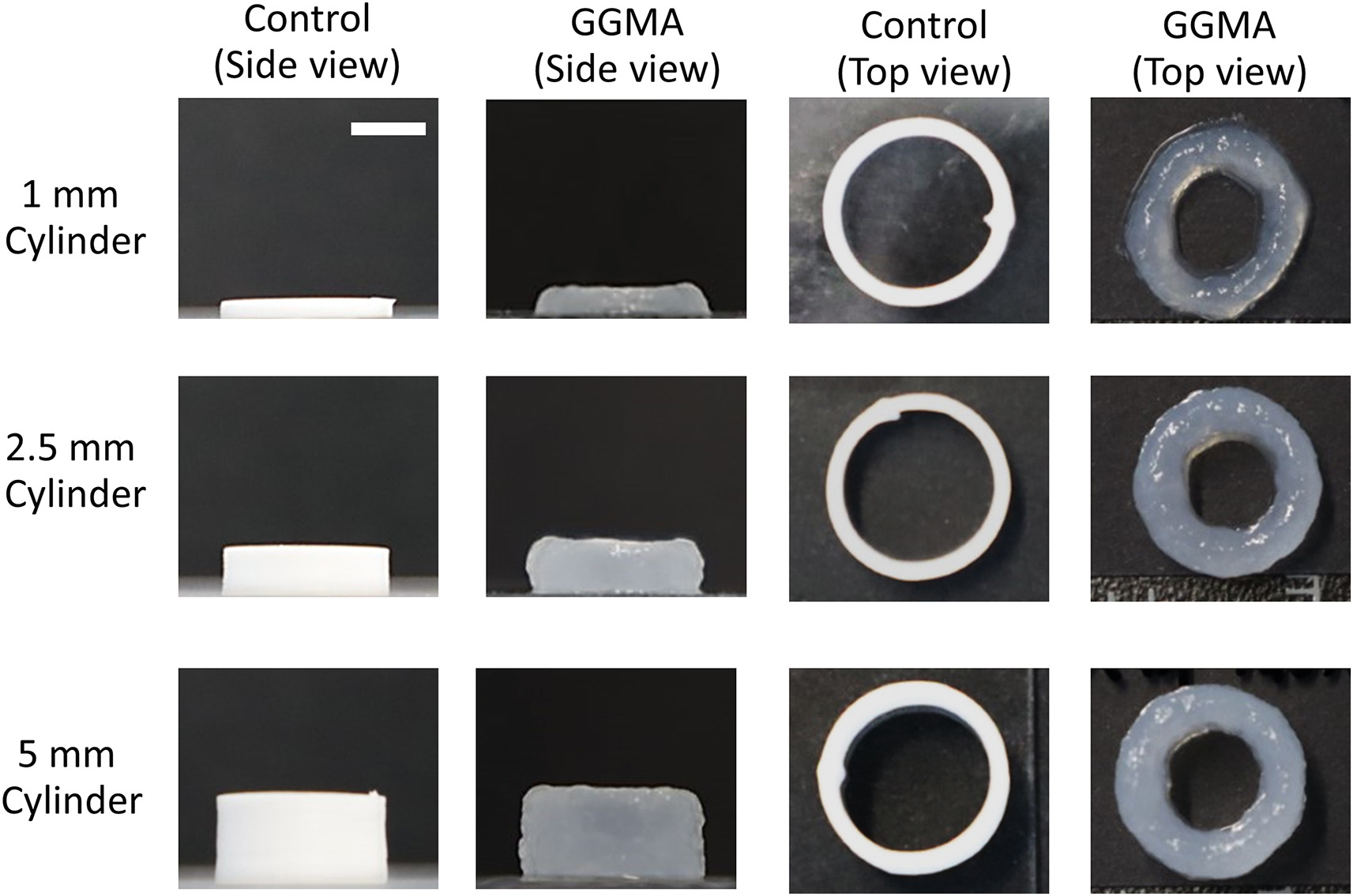
The optimized GGMA ink was 3D printed with consistent fibers and demonstrated high printability. The resulting 3D printed structures, while still lacking high resolution compared to the control structures used in the study, demonstrated high accuracy, shape fidelity, structural integrity, and mechanical stability.
According to the team, the study proved their two-step crosslinking technique as an effective approach to turn GGMA inks into 3D printable bioinks which could have a wide range of potential bioprinting applications.
Further information on the study can be found in the paper titled: “Two-step crosslinking to enhance the printability of methacrylated gellan gum biomaterial ink for extrusion-based 3D bioprinting,” published in the Bioprinting journal. The study is co-authored by H. Jongprasitkul, S. Turunen, V. Parihar, and M. Kellomaki.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows side-views and top-views of printed cylinders for the evaluation of printing accuracy and structural integrity. Image via Biomaterials.



