The impact of pandemic continued to dominate the headlines in the 3D printing industry during September. Whether it be the cancellation of industry events, or the efforts of additive manufacturers to help fight the disease, COVID-19 was never far from the news.

COVID-19 forces Formnext reshuffle
Messe Frankfurt confirmed that the formnext was going online-only. And the trade show was held in the guise of Formnext Connect, the industry missed out on the opportunity to network and catch-up in its customary way.
3D printed test swabs, meanwhile, have played a vital role in combating COVID-related equipment shortages, and PrintParts revealed in September that PostProcess was aiding its efforts in New York City. Leveraging PostProcess’ DEMI resin removal solution, the company was able to fabricate five times as many tests, eventually producing over 1 million kits.
“Pivoting our production business from low-to-mid volume parts for aerospace, defense and industrial manufacturing, to a high volume medical device under extremely tight deadlines, was a huge challenge,” Robert Haleluk, Founder of PrintParts said at the time. “We couldn’t have done it without the PostProcess DEMI.”
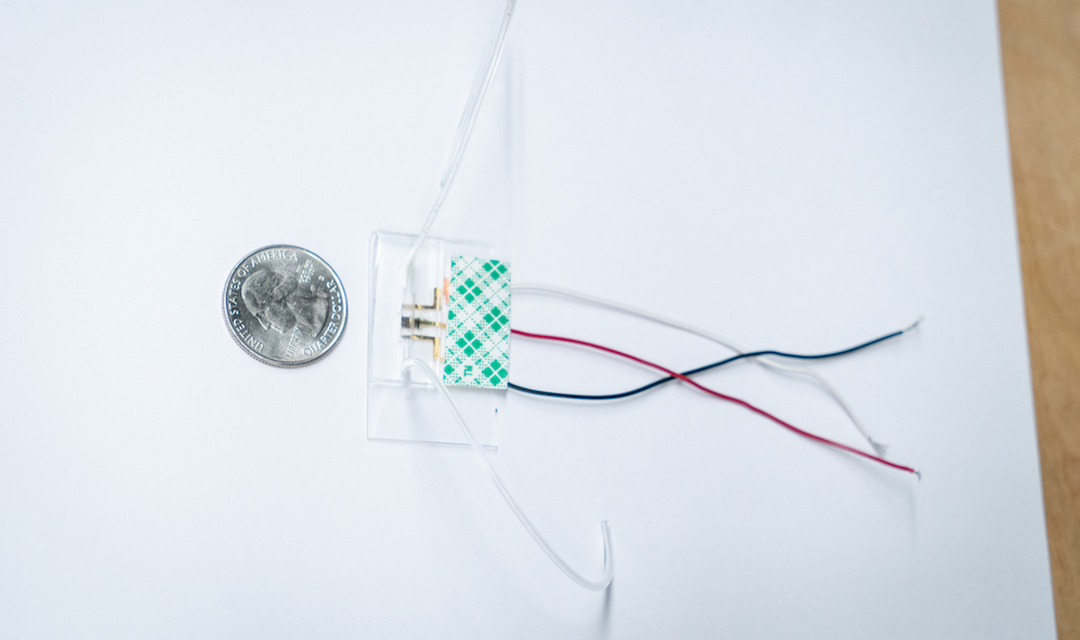
Elsewhere, scientists at Carnegie Mellon University (CMU), developed a novel 3D printed sensor, that proved capable of identifying COVID-19 antibodies in just 10 seconds. The low-cost reusable device, which was created using Optomec’s Aerosol Jet Printing (AJP) technology, could also be used to diagnose Ebola or HIV in the future.
The pandemic also had a significant impact on the financials of several 3D printing firms, and GE’s figures revealed that it wrote-off $877 million in goodwill impairment charges during Q3. GE wouldn’t comment on its decision to devalue some of its portfolio, but the charges are thought to relate to its acquisitions of Arcam and Concept Laser in 2016.

Advances made in additive workflow software
Several 3D printing software developers announced advances in their process management programs during the month, starting with VoxelDance, which launched its Additive 2.0 data preparation platform. The new iteration of the app featured an upgraded model repair algorithm, which was capable of ‘plugging up’ holes within 3D models.
Following the release of VoxelDance’s update, Metal additive manufacturer Sintavia announced a partnership with Siemens, which will see it develop software for its Xcelerator portfolio. As part of the deal, Sintavia will gain early access to Siemens’ new program, and provide technical feedback based on its performance.
“We’re excited to be collaborating with Siemens to help make industrialized additive manufacturing a reality,” said Brian Neff, CEO of Sintavia. “The end-to-end solution that Siemens has been developing is absolutely essential to making additive a viable manufacturing process.”
Similarly, workflow software company Authentise revealed that it had started working with AMES system developer Addiguru. Working together, the firms intend to integrate in-situ monitoring functionality into AMES, allowing for changes to be made during the process in real-time, alongside the digitized workflow management that’s currently offered.
Neural and renal research progresses
September proved to be an especially successful month for scientists in the field of psychological diseases, as 3D printing helped several of them to develop breakthrough treatments. Engineering firm Renishaw, for instance, gave further details of joint research with Herantis Pharma that yielded an additive neuroinfuse drug delivery device.
During human trials, the device demonstrated the ability to administer repeated infusions into an organ’s functional tissue, making it a potential treatment for Parkinson’s disease. Researchers from the University of Sheffield also made progress with a 3D printed neural implant in September that could be used as a medication for paralysis sufferers.
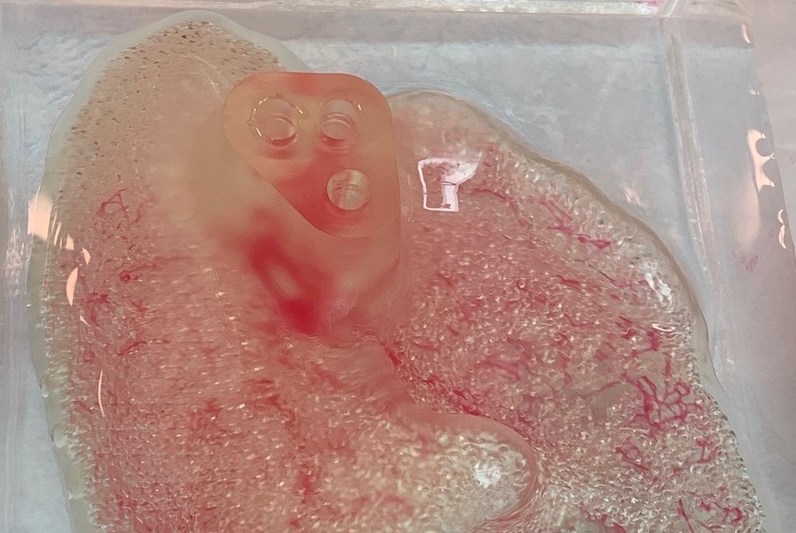
The team’s novel device acted as a link between the human brain and a computer in a way that could be leveraged to combat various neurological conditions. Finally, on a more commercial note, biotechnology firm United Therapeutics agreed to broaden its partnership with CollPlant, so that the firms put their 3D printed kidneys into production.
While the companies are still preparing to go into serial manufacturing with their additive organs, they have turned a former tobacco complex into a modern 3D bioprinting factory.
“Organ shortages are an unmet global health need,” said Yehiel Tal, CEO of CollPlant. “By partnering with United Therapeutics for the past couple of years, we have made significant progress with this pivotal organ manufacturing initiative.”
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows the COVID-19 nasal testing swabs that were fabricated by PrintParts in collaboration with PostProcess. Photo via PostProcess.


