A team of researchers from Washington State University’s School of Mechanical and Materials Engineering have developed a novel, customizable 3D bioprinting material designed to better mimic the structure of natural tissue.
With programmable mechanical properties, the scaffolding material is better equipped to encourage natural cell growth in a variety of environments, providing scientists with a more reliable route to custom tissue fabrication. Professor Arda Gozen – a co-author of the study – sees a bright future for the material as an easy-to-use bioink. He believes that doctors may well be able to hit a button, bioprint a scaffold with the material, and produce replacement skin, cartilage, and other bio-structures for patients on demand.
“The success of this method in manufacturing functional tissues relies heavily on how well the fabricated structures mimic the native tissues,” explains Gozen. “If you want to grow cells and turn them into functional tissue, you need to match the mechanical environment of the native tissue.”

The art of 3D bioprinting
The holy grail of 3D bioprinting is the fabrication of entire replacement organs, a milestone that would eliminate transplant wait times and take a huge load off healthcare systems worldwide. As it stands, 3D bioprinting technology is only capable of producing simple tissue samples, whereby bioinks are deposited layer by layer to erect scaffolds, which then provide a nurturing environment for cells to grow in.
Unfortunately, evolution is significantly more complex than our high-precision micro-deposition systems, meaning real biological cells often have a difficult time growing on man-made scaffolds. For example, skin cells like to grow on scaffolds that feel like real skin, while muscle cells like to grow on scaffolds that feel like real muscle.
The art of 3D bioprinting is largely concerned with duping these biological cells into thinking they are surrounded by the real thing. A typical way in which researchers fine-tune their scaffolds is by adding or removing trusses, which increases or decreases the stiffness of the structure. While the approach is relatively simple, it doesn’t quite provide the flexibility needed in end-use tissue engineering.
Gozen adds, “We don’t have a lot of knobs to turn. You need more degrees of freedom – to create something softer or harder without changing the structure.”
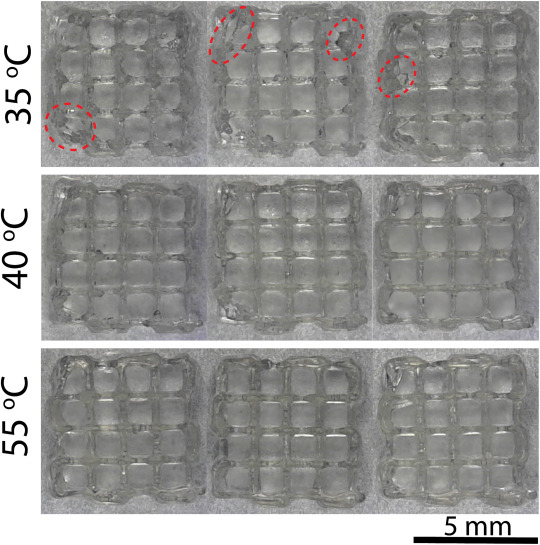
An adaptable 3D bioink
To address the need for adaptability, the Washington team developed a novel bioink with customizable mechanical properties. Created using gelatin, gum Arabic, and sodium alginate, the bioink combines three chemical processes to ‘tie’ the three ingredients together, almost like a braided rope. Modifying the separate chemical processes enables the scientists to fine-tune the stiffness of the final scaffold, without having to modify the geometric design with trusses.
“That gives you the capability of tuning the properties without changing the scaffold design and gives you the additional degree of freedom that we are seeking,” explains Gozen.
While the work is still in its early stages, Gozen’s team has already used the material to 3D print nose cartilage scaffolds. Simulating the complexity of natural tissue remains a challenge, but with varying print parameters or ingredient compositions, the team believes it can eventually fabricate usable scaffold structures for larger-scale tissue engineering applications.
“You’re not assembling Legos here. It’s always about replicating nature that works with the body,” concludes Gozen. “You can make living structures, but they look nothing like the native tissue. Precision is key because there is no single mechanical property target for a single piece of tissue.”

Further details of the study can be found in the paper titled ‘3D printed, mechanically tunable, composite sodium alginate, gelatin and Gum Arabic (SA-GEL-GA) scaffolds’. It is co-authored by Mahmoud Amr, Arda Gozen et al.
In a similar vein, researchers from Pennsylvania State University have previously developed a 3D bioprinting process capable of printing hard and soft tissue at the same time to repair skin and bone injuries. Using two specifically-engineered bioinks and bioprinting processes, the team was able to repair a hole in the skull and skin of a rat model within minutes in a single procedure.
Elsewhere, scientists from the King Abdullah University of Science and Technology recently developed a new method of 3D printing hydrogel scaffolds based on ultrashort peptides. The team used the short amino acid chains to formulate a bioink for tissue engineering purposes.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows Professor Arda Gozen in the bioprinting lab. Photo via WSU.


