Researchers from the Stevens Institute of Technology in New Jersey are using computational modeling techniques to advance microfluidics-based 3D bioprinting, and they hope it can one day enable the fabrication of entire human organs.
Many of today’s state-of-the-art bioprinters are based on extrusion processes, whereby bioinks are deposited by a nozzle to create tissue structures measuring around 200 microns.
A bioprinter based on microfluidics would instead operate by precisely manipulating liquids through tiny channels, and could print structures measuring just tens of microns across. This is more akin to the single cellular scale and is reportedly what’s needed if we’re ever to make 3D printed organs a reality.
Robert Chang, the associate professor at Stevens’ Schaefer School of Engineering & Science who’s leading the work, explains, “The scale is very important, because it affects the biology of the organ. We’re operating at the scale of human cells, and that lets us print structures that mimic the biological features we’re trying to replicate.”

Towards human organ transplants
Organ transplants can be a life-saver for individuals suffering from serious illnesses, but there’s always been a shortage of suitable donors. In the US, there are currently more than 100,000 patients on the transplant waiting list, with around 17 dying each day while waiting for a donor.
3D bioprinting technology has long been heralded as a potential solution to the problem, but a lack of technological development means we aren’t quite there yet. As it stands, bioprinters are adept at fabricating simple single-celled tissues and structures, but Chang and his team believe microfluidics may be the key to engineering virtually any type of complex tissue. This includes entire vital organs and even skin printed directly onto open wounds.
“Creating new organs to order and saving lives without the need for a human donor will be an immense benefit to healthcare,” said Chang. “However, reaching that goal is tricky because printing organs using bio-inks — hydrogels laden with cultured cells — requires a degree of fine control over the geometry and size of printed microfibers that current 3D printers simply can’t achieve.”
As well as allowing for much smaller scales, a microfluidics-based process will also be compatible with multiple bioinks. Each of these bioinks might contain precursors for different cell types, so users would be able to combine them within a single printed tissue structure. This is crucial for complex organs such as livers and kidneys, as they rely on a wide variety of cell types working in tandem to operate.
Computationally modeling 3D bioprinting
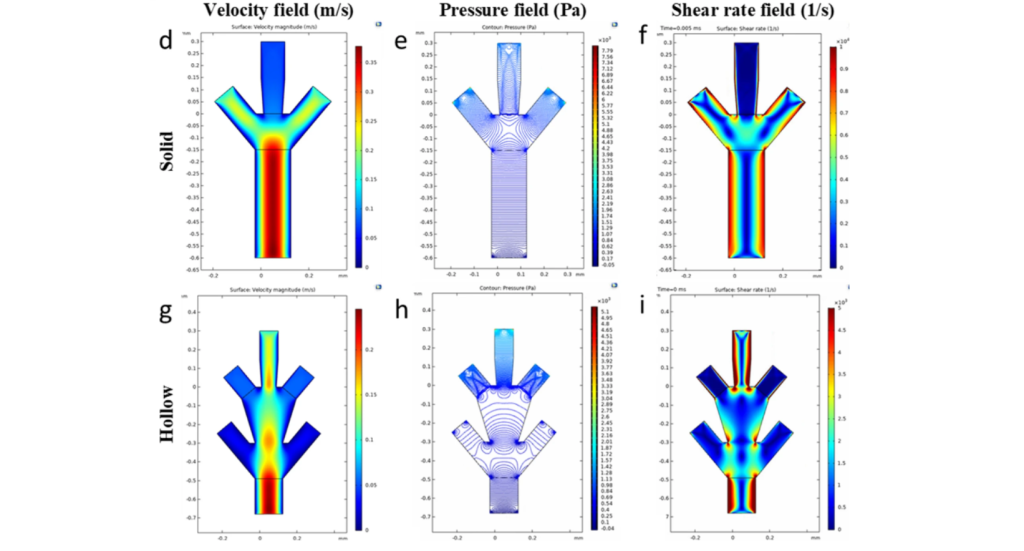
Scaling the 3D bioprinting process down to just tens of microns is no easy feat, and requires studying how various parameters such as flow speed, channel structures, and fluid dynamics affect the properties of the printed tissue structures. To do just that, Chang and his team developed a computational model of a microfluidic printhead. The model allows them to fine-tune parameters and predict how they might affect the process without having to conduct tedious physical experiments.
Ahmadreza Zaeri, first author of the study, stated, “Our computational model advances a formulaic extraction that can be used to predict the various geometrical parameters of the fabricated structures extruded from the microfluidic channels.”
By simulating the outcomes of real-world experiments, the team is now gaining a better understanding of how a variety of organ structures might be printed. The results will be used to develop multiple-cell-type bioinks. Chang is also working on adapting microfluidic bioprinting to fabricate skin directly onto wounds.
Further details of the work can be found in the paper titled ‘Numerical analysis on the effects of microfluidic-based bioprinting parameters on the microfiber geometrical outcomes’.
This certainly isn’t the first time microfluidic technology has made headlines in the additive manufacturing sector. Earlier this year, Phase Inc, a North Carolina-based medical 3D printing startup, partnered with Virginia Tech to advance the field of microfluidics 3D printing. Together, Phase and Virginia Tech will use the former’s proprietary LE3D printing technology to develop novel microfluidic devices that will help researchers formulate new and improved medical treatments for conditions such as brain cancer.
Elsewhere, researchers from the University of Bristol have previously developed a novel, low-cost, and open-source 3D printing process for producing microfluidic devices. Requiring only simple domestic equipment and a standard desktop 3D printer, and having been developed in free-to-use software, the researchers’ process reduces the cost and complexity of fabricating microfluidics to make the field more accessible.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter, liking us on Facebook, and tuning into the 3D Printing Industry YouTube Channel.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows BICO’s Bio X 3D bioprinter, an extrusion-based system. Photo via BICO.



