Renishaw additive manufacturing has played a pivotal role in the recent clinical trial of an experimental treatment for Parkinson’s Disease. Documented in a two part-series produced by the BBC titled The Parkinson’s Drug Trial: A Miracle Cure? this trial includes the novel development of 3D printed medical device capable of delivering drugs directly to affected parts of the brain. After 40-80 weeks in-situ, the implants have become part of a potentially landmark advance in the treatment of this long-term degenerative disorder.
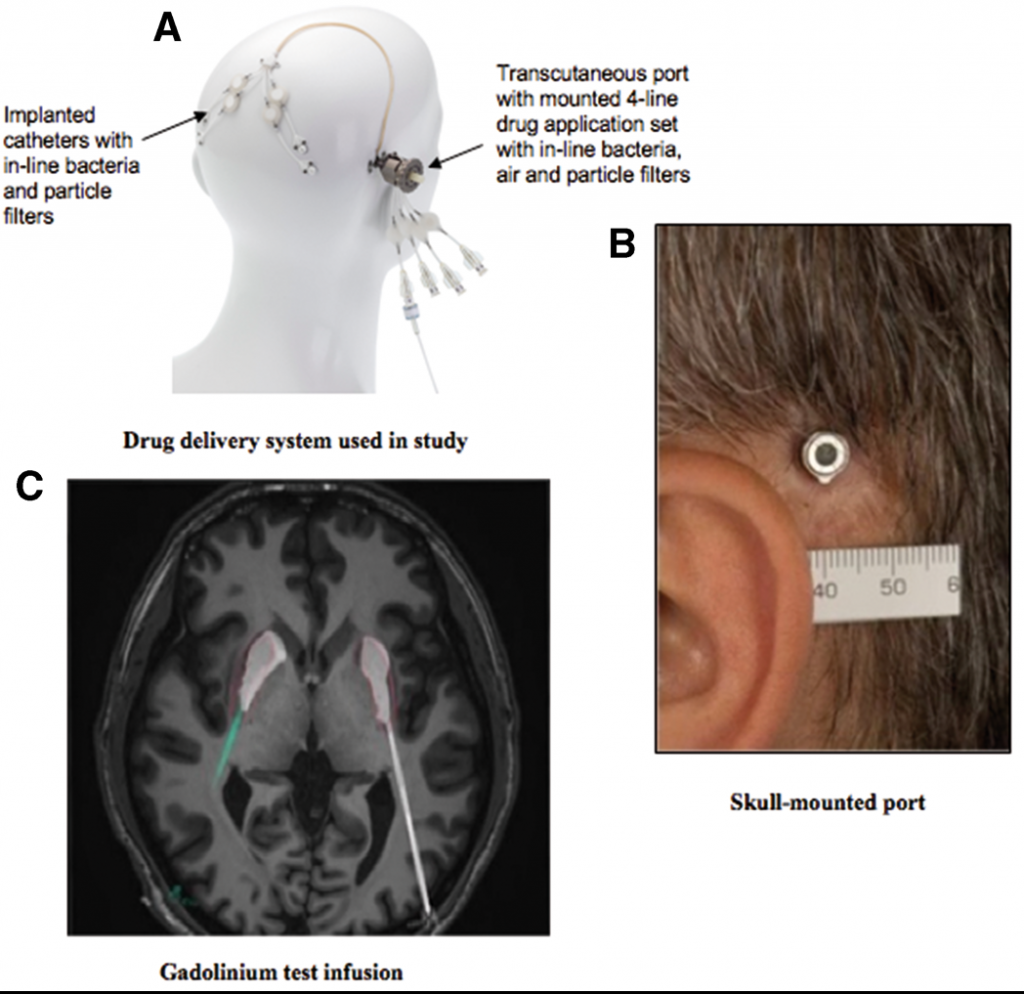
Professor Steven Gill, trial lead and Consultant Neurosurgeon for North Bristol NHS Trust, comments, “This trial has shown that we can safely and repeatedly infuse drugs directly into patient’s brains over months or years through a small implanted port that emerges through the skin behind the ear.”
Paul Skinner, General Manager for Neurological Products at Renishaw, adds:
“This provides great potential for using the drug delivery system, being developed by Renishaw, for future Parkinson’s studies and experimental treatments for other neurodegenerative diseases and brain tumours.”
Transcending the blood-brain barrier
The drug candidate used in this trial is GDNF, a protein growth factor critical to the development of the brain’s synapses. It was discovered in 1952 in Nobel-Prize winning research from Rita Levi–Montalcini and Stanley Cohen, and over subsequent decades went on to become the topic of a number of pre-clinical and clinical trials for its application as a therapeutic treatment for regenerating brain cells.
In this process, its was discovered that GDNF administered intravenously did not effectively reach the affected cells of the brain. This is due to the protective blood-brain barrier (BBB) which is also, as a result, the subject of some 3D biopriting studies. The solution, as discovered by Professor Gill, was to introduce GDNF through catheters on the brain that permeate the BBB.
The £3 million Phase 2 study of this trial method, as detailed in the BBC documentary, was funded by Parkinson’s UK with support from the Cure Parkinson’s Trust. It is the subject of a paper published in the Brain journal of neurology, and a subsequent article which will discuss the results of an open-label extension study.
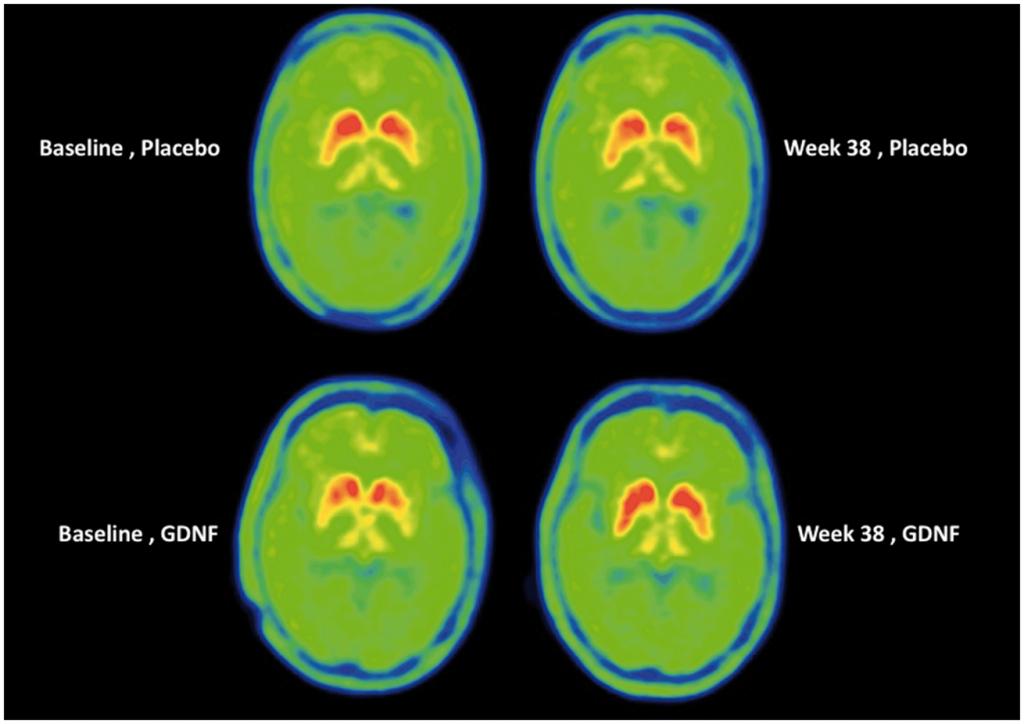
The possibility of restoring motor function
In order to administer GDNF in such a direct way, Renishaw was drafted in to develop a specialist device that could be implanted into a patient’s skull over a long period of time. With expertise in maxillo-facial implant production, and a number of other recent medical successes, it is clear why Renishaw was the perfect choice for this project.
The end result of the collaborative R&D between Renishaw, NHS specialists, and other professional and academic partners, was a 3D printed titanium port for the drug’s input.
Over the course of 40 weeks, this port was used to deliver a controlled dosage of GDNF to a select group of patients. At the end of this period, when compared with patients receiving a placebo drug, the trial did not achieve its intended result – i.e. an improvement in motor function scores. However, following this, the team extended the study for a further 40 weeks, giving the drug to both groups of patients. After this extension, the study found a 30% improvement in motor scores. Further, the group that had received GDNF dosage for 80 weeks in total had a 100% improvement in the posterior portion of the putamen – the region of the brain associated with motor function.
“Even at a low dose we have seen evidence of patient improvement, which is incredibly encouraging,” says Professor Gill. “Now we need to move towards a definitive clinical trial using higher doses and this work urgently needs funding.”
“I believe that this approach could be the first neuro-restorative treatment for people living with Parkinson’s which is, of course, an extremely exciting prospect.”
Further reading and viewing
Full results of the first 40 week study of GDNF are published open access in the paper “Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease.” In addition to experts at Renishaw and specialists from North Bristol NHS Trust, the paper has credited co-authors from the University of British Columbia, Cardiff University and Canadian biopharmaceutical company MedGenesis Therapeutix Inc. Results of the open-label extension study will be reported separately. A similar device produced by Renishaw called ‘neruoinfuse’ is also now been investigated for its potential in other clinical trials.
The final part of the TV series The Parkinson’s Drug Trial: A Miracle Cure? is due to air on BBC Two the UK on Thursday March 7, 2019, at 9pm GMT. In this episode, researchers will reveal the results of the trial and examine the treatment’s potential global impact.
For more medical 3D printing updates subscribe to our newsletter, follow us on Twitter and like us on Facebook. Visit 3D Printing Jobs for new opportunities in your area.
Featured image shows the drug delivery device as used in the GDNF trial. Image by Chris Marshall/Mint Motion



