Biotechnology firm Prellis Biologics has announced the closure of a $14.5 million Series B funding round alongside the creation of a novel antibody discovery platform.
Effectively a functioning immune system in a dish, the firm’s platform is capable of recreating human cell-to-cell interactions and immune responses, making it ideal for conducting disease therapy R&D. To support the continued evolution of these bioprinted tissues, the company has also gained substantial backing from new investors, who are said to be “extremely excited” about their potential.
“The ‘EXIS’ platform offers unprecedented in-vitro access to a functional human immune system,” said Dr. Melanie P. Matheu, CEO of Prellis Biologics. “We’ve built a platform that enables promising science to be rapidly advanced into new patient therapies. We’re thrilled to continue expanding upon the exciting potential of this technology, thanks to the support of our investors and collaboration partners.”
Multiphoton Holographic technology
Based in San Francisco, Prellis Biologics specializes in the bioprinting of vascularized channels that serve as a basis for creating functional human tissues. Primarily, the company is able to achieve this thanks to its Multiphoton Holographic technology, an approach that’s similar to conventional vat polymerization, in which lasers are used to cure materials infused with live cells at a high speed and resolution.
In the past, Prellis Biologics has sought to expedite drug R&D and commercialize its technology by integrating it into CELLINK’s Holograph-X bioprinter. At the time, the resulting system was said to be capable of delivering nutrients to scaffolds more efficiently, better supporting their growth into tissues, thus “empowering customers to advance their research in the field of 3D bioprinting human organs.”
Prellis Biologics itself has also targeted bioprinted organoids before, setting out to produce a human kidney vascular system inside of 12 hours in 2018, as a means of overcoming the cell deterioration hindering existing research, and it raised $8.7 million a year later, to further its experiments into the transplantation of bioprinted cells into a living animal model.

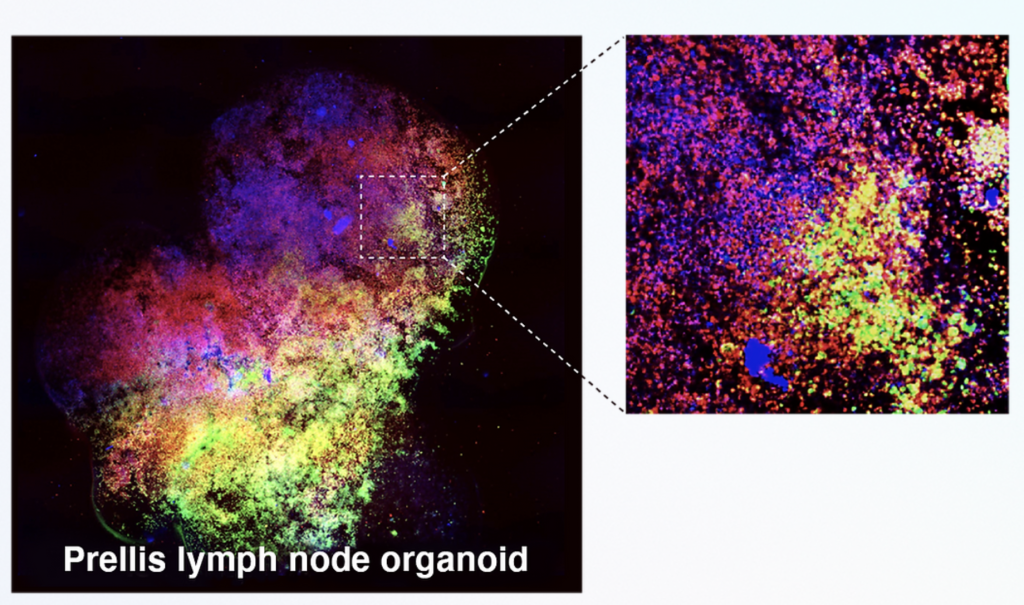
An ‘Externalized Immune System’
Using its proprietary 3D bioprinting technology, Prellis Biologics now appears to have shifted its focus away from attempting to create vascularized scaffolds, setting out to engineer more complex lymph node organoids instead, and following extensive research in this area, the firm has unveiled a breakthrough with its Externalized Immune System or ‘EXIS.’
Believed to be the first of its kind, Prellis Biologics’ EXIS is said to be capable of achieving interactions such as B cell class-switching and somatic hypermutation that are key to developing antibodies. The platform is also able to break cells’ natural failsafes, which often prevent auto-antibody development, thus it could now expedite the R&D of therapies for illnesses like cancer or autoimmune disease.
Already, Prellis Biologics says it has used EXIS to target antigens in various formats to produce human antibodies with high affinities, sequence diversity and potency, within a period of just 3 weeks. As a result, the firm believes that its platform has the potential to “significantly reduce the discovery and lead identification time” of existing in-vivo or in-vitro drug screening and vaccine testing methods.
To fund the ongoing development of its organoids, the company has also gained fresh investment, taking its total to $29.5 million, in a round led by Celesta Capital and backed by Khosla Ventures. As part of the financing deal, Celesta Capital’s Michael Marks and ex-Berkeley Lights COO Shaun Holt, have agreed to join Prellis Biologics’ board and lend their expertise to guide its future growth.
“Today, pharmaceutical antibody discovery takes a long time, carries high costs due to reliance on animals, and the antibodies too rarely perform as hoped once they reach human trials,” added Marks. “We are ready to support marketing for the EXIS platform now that initial deals have helped to prove its great potential.”

3D bioprinting’s therapeutic potential
As the cellular viability of 3D printed tissues continues to advance, so do their potential applications as a means of testing the efficacy of novel drugs, therapeutics and even vaccines. Since last year, for instance, HK inno.N has been working with T&R Biofab to 3D bioprint tissue test models for the evaluation of new autoimmune and skin disorder remedies.
Scientists at the Chinese Academy of Sciences and University of Science and Technology of China, have even managed to use bioprinting to develop a way of ‘curing’ untreatable spinal cord injuries. Leveraging a novel bio-ink, the Chinese team created neural stem cell-loaded tissues in July 2021, capable of carrying instructions via impulses from the brain, similar to those seen in living organisms.
More recently, regenerative medicine specialist CTIBIOTECH has leveraged its 3D bioprinting technology to produce a unique colon cancer treatment platform. Developed alongside the Medical University of Plovdiv and UMHAT-Eurohospital, the tissues are designed to yield reproducible human colon cancer disease models for chemotherapeutic screening applications.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
For a deeper dive into additive manufacturing, you can now subscribe to our Youtube channel, featuring discussion, debriefs, and shots of 3D printing in-action.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows a high-resolution image of Prellis Biologics’ 3D bioprinted Externalized Immune System. Image via Prellis Biologics.



