This year saw notable breakthroughs in the medical sector, including a new application in 3D printing for hair follicle research by researchers at US-based Rensselaer Polytechnic Institute (RPI).
3D Printing Industry interviewed Pankaj Karande, Associate Professor, Chemical and Biological Engineering at RPI to learn more about this novel study.
The research, also involving lead author Carolina Catarino, and collaborative partner Grupo Boticario explores the potential for in vitro studies to advance molecules promoting hair growth and improving transplantation success. This innovative approach holds promise for expediting complex skin graft development, particularly benefiting regenerative medicine applications such as burn victim treatment.
“It is tempting to think that we have now been able to develop a solution for hair restoration. Translation of our proof-of-concept studies to demonstrating that we can form fully-grown hair in human skin will require additional research and optimization. Not to mention regulatory considerations in translating this research to human patients. Our studies have made important contributions but there is still a lot of work ahead,” says Professor Karande.
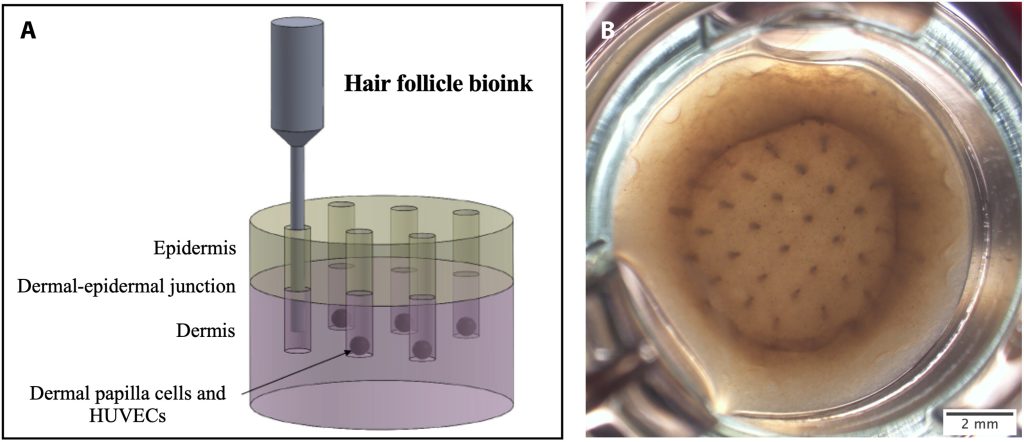
Bioprinting of functional hair follicles in skin constructs
Professor Karande explains that to produce the bioprinted skin sample, the initial step involves designing bioinks composed of biomaterials such as proteins and cells. Proceeding with patient consent, the team sourced these cells from discarded skin samples either isolating them in-house or purchasing them commercially. The isolation process takes approximately two weeks, after which the cells undergo a cultivation and expansion phase in the laboratory lasting 1 to 4 weeks to increase their numbers. Subsequently, the cells are combined with proteins and other biomaterials to formulate the bioinks.
The bioinks are then loaded into the bioprinting equipment which is very similar in concept to the conventional 3D printers. Instead of using inks made of melted plastic, polymers, or resins, the team utilizes cartridges filled with biological material. Once loaded, a pre-programmed algorithm guides the deposition of each material, creating a 3D tissue. “The process starts with the deposition of the bottommost layer (dermis), primarily composed of collagen I and dermal cells. After the dermis gels, the printing progresses to include hair follicles and the external skin layer (epidermis). This entire printing process for 12 samples (each about an inch in diameter) typically takes around 1 hour,” he said.
Following printing, the samples undergo incubation in a controlled environment with regular media changes, maintaining optimal temperature and CO2 levels. This approximately 2-week incubation period allows the tissue to mature to its final stage. Subsequently, the bioprinted skin samples undergo in vitro substance evaluations, signifying a major advancement in diverse tissue engineering techniques.
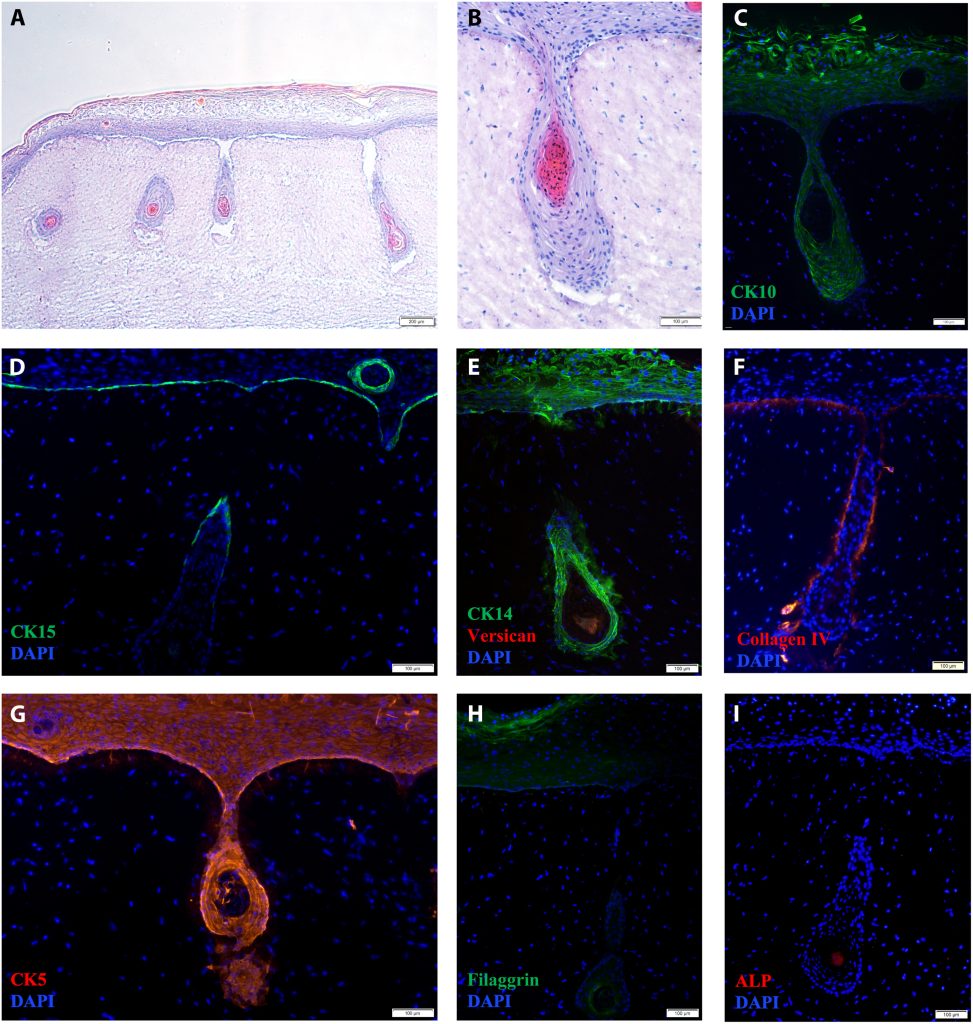
Revolutionizing hair follicle regeneration
He further explained that reconstructing hair follicles using human-derived cells is challenging due to the loss of stem-cell-like characteristics outside their natural environment. Cells struggle to generate new follicles without this context. Research suggests that culturing them in a three-dimensional environment partially restores their capacity, potentially forming new follicles or shafts. To address this challenge, the team’s unique approach utilizes bioprinting technology to recreate the three-dimensional environment, facilitating the recovery of cell inductive capacity in skin model reconstruction with hair follicles, according to the Professor.
From a technical standpoint, the main challenge in realizing the project involved integrating diverse cell types into designated bioinks and aligning hardware and software to build functional human tissue. Coordinating these elements posed a significant technical hurdle for the team, highlighting the complexity of advancing hair follicle regeneration.
“When we talk about in vitro models for efficacy and toxicity testing of compounds, we do not expect any side effect of having hair follicle. The inclusion of such structure could enhance our predictivity capacity to understand the effect of a compound since the model would be more physiologically relevant and allow us to study other biological process such as hair growth,” he says. Regarding the potential use of these models for regenerative medicine, there is always the inherent risk of rejection by the body such as in any transplant. In the future, the team plans to potentially combine the technology used to produce the models with the use of patient-derived cells using iPSCs cells which can reduce the chance of rejection.
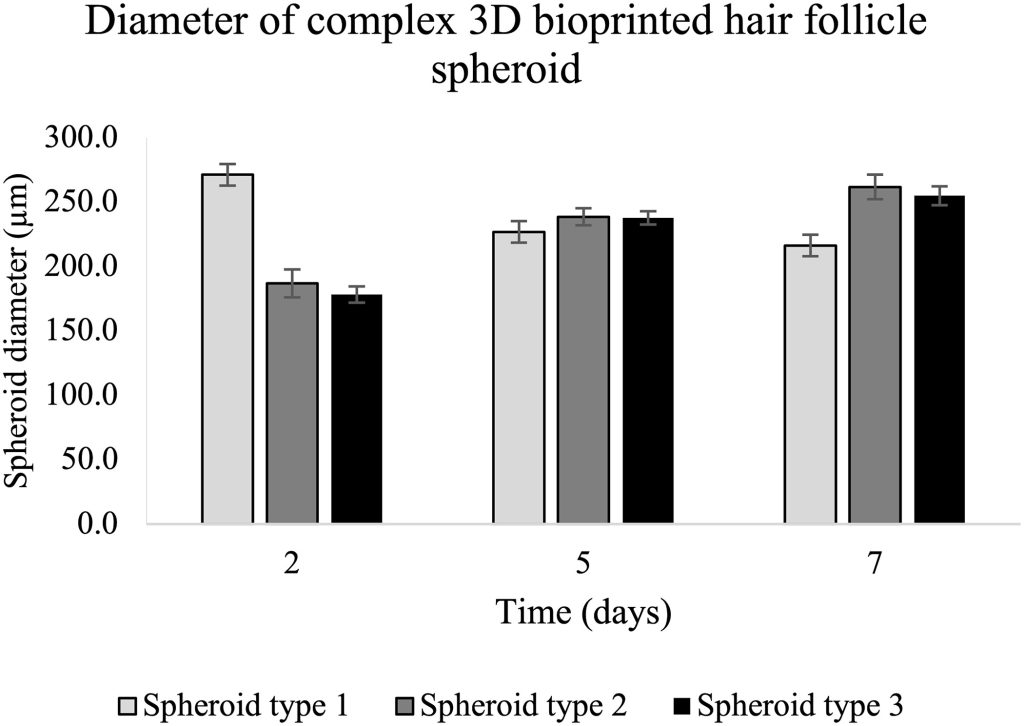
Navigating bioprinting: customization and biological realities
Various bioprinting technologies, including extrusion, inkjet, laser-assisted, and stereolithography, have been explored by researchers. Each technology demands customization in printing protocol, bioink composition, cross-linking mechanism, and curing. The combination of bioinks, bioprinter, and printing program enabled the team to design a 3D model within relevant limitations, says the Professor.
“However, even though such technology allows us to increase the complexity of what is being created in the laboratory we still rely on biology to do much of the work. We can define the hair density we wish to print and even direct the growth direction; however, hair color and fiber curvature are mostly defined by the DNA within the cells,” explained Professor Karande.
He further explained that currently, the models developed are produced in a laboratory under controlled conditions of temperature, humidity, and without direct sunlight exposure. Moving forward in the development of these models, environmental factors such as sunlight exposure as well as different humidity conditions and application of substances will be evaluated to test the robustness of the model.
Optimizing skin model complexity for future applications
The presented work is a proof of concept, demonstrating the efficiency of 3D bioprinting in enhancing the complexity of reconstructed skin models. While not yet ready for commercialization, the team is actively refining the printing protocol and improving media composition for an extended culture period. Current bioprinters can produce multiple tissues or mini organoids of hair follicles within an hour, and as technology evolves, throughput is expected to increase. The focus remains on advancing the technique and optimizing scalability for future applications.
The Professor explained that the team’s objective is to optimize conditions for sustained growth and maturation. They express interest in assessing the viability of the engineered tissue, particularly the hair follicles, to grow hair shafts when grafted onto an animal model. The next step involves transitioning to clinical tests before the technology can be brought to the market.
“We are interested in exploring the potential of our model for in vitro testing of different topical compounds and their potency or toxicity for various medical and cosmetic applications. For this case, once these optimizations and in vitro validations have been performed, the model could be commercialized. Stay tuned for forthcoming updates as we aim to share our progress and findings through publications in the near future,” concluded Professor Karande.
Read all the 3D Printing Industry coverage from Formnext 2023.
What does the future of 3D printing for the next ten years hold?
What engineering challenges will need to be tackled in the additive manufacturing sector in the coming decade?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows from left to right: Pankaj Karande and Carolina Catarino. Photo via RPI.



