German medical device company endocon GmbH has developed a 3D printed surgical tool with the help of GE Additive Concept Laser Direct Metal Laser Melting (DMLM) technology. The medical-grade device is designed to improve and simplify the removal of hip implants, with the optional use of fifteen additively manufactured stainless steel blades.
With this technology, Endocon has been able to save time and money on manufacturing while simultaneously reducing the risk of complications.

Simplifying surgical removal of hip implants
The implantation of acetabular cups is a relatively routine hip replacement operation. Their removal, however, is a much more complicated process. Typically, surgeons use a chisel to dislodge the implanted cup, presenting risks to a patient’s bone and soft tissue.
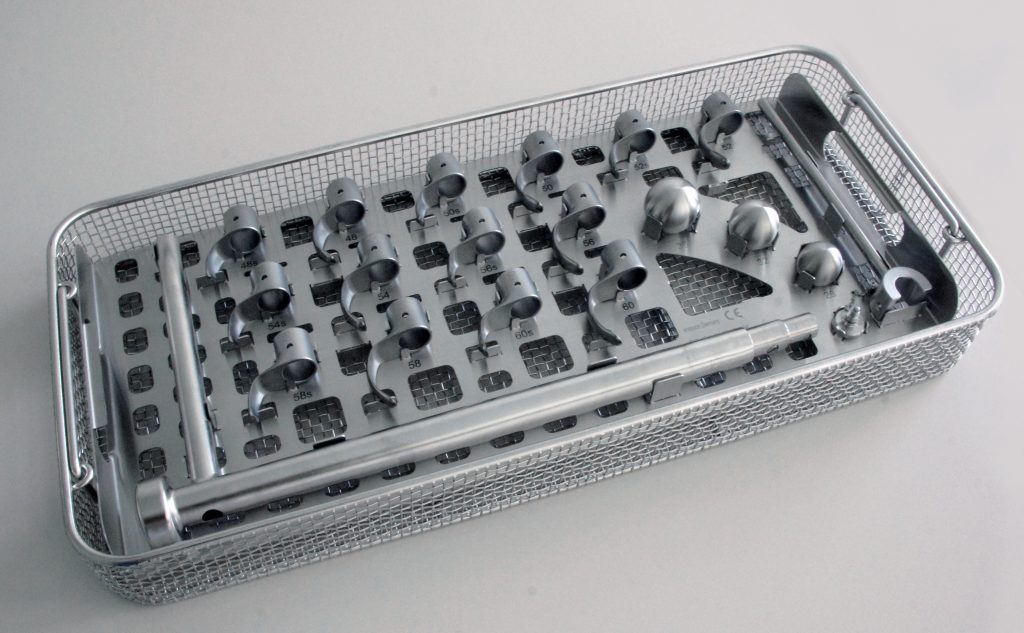
Endocon’s endoCupcut product was created as an alternative cup removal tool. With fifteen interchangeable blades in various sizes ranging from 44mm to 72mm, the endoCupcut tool provides more precise cutting along the edge of an acetabular cup for loosening and extraction.
These blades were made on a Concept Laser Mlab cusing 100R machine at Weber-KP an additive manufacturing, 3D scanning and engineering service bureau.
3D printed blades
17-4 PH stainless steel powder was used as the material to make blades for the endoCupcut device. Depending on their size and orientation, between two and six blades can be 3D printed at any time on the Mlab cusing 100R, on a build plate of 90 mm x 90 mm. Including data preparation, 3D printing, high-quality surface finishing, hardening and bead blasting, it takes Weber-KP approximately three weeks to deliver a complete set of endoCupcut blades.
Before additive, these blades would have been cast in metal from a mold. In addition to issues with reputability, corrosion and consistency, casted blades would have a lead time of up to three and half months for production.
In terms of biocompatibility, the 3D printed blades led to a more consistent outcome of hip cup replacement, and the rejection rate was reduced from 30% to under 3%.
Klaus Notarbartolo, general manager at endocon, comments, “We’ve also been able to reduce the cost per blade by around forty to forty-five percent. That means cost savings for us and in turn for our customers. When you combine that with a reduction in product development time, higher efficiency and lower rejection rates, then the business case for additive really becomes attractive.”
GE Additive in medicine
The endoCupcut device adds to a growing list of medical applications from GE Additive. In June 2018, UK-based medical technology company Sutrue launched its automated suturing device made with gears 3D printed by Concept Laser. And the GE Additive ARCAM Q10 Plus has acquired FDA clearance for the manufacture of orthopedic implants.
For more of the latest 3D printing in healthcare news subscribe to the 3D Printing Industry newsletter, follow us on Twitter and like us on Facebook.
Looking for a change of pace? Sign up for 3D Printing Jobshere.
Featured image shows the endoCupcut device with a successfully removed hip cup. Photo via GE Additive



