A 3D printed biomimetic graft developed by a multidisciplinary team at Harvard University’s Wyss Institute led by Gliklich Healthcare Innovation Postdoctoral Research Fellow Nicole Black is seeking 510K clearance from the US Food and Drug Administration (FDA).
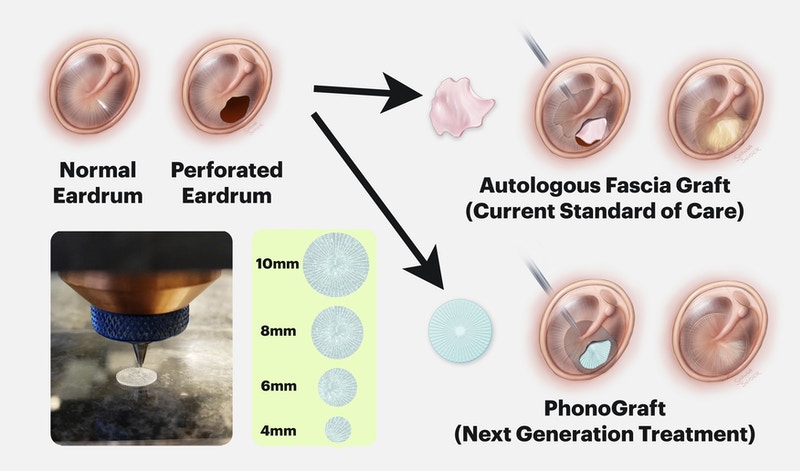
Named the PhonoGraft, the device is designed to mimic the human body’s eardrum and can be used in patients who suffer ear trauma to enable the eardrum to heal itself by generating new cells.
It is hoped that the 3D printed device will improve the speed and success of surgical procedures for eardrum repair, while lowering the cost of the procedure for patients.
Developing the PhonoGraft
Perforations of the eardrum as a result of trauma, chronic ear infections, and abnormal skin growth can result in severe hearing loss and recurring infections, and according to the Wyss Institute occur in around 30 million people worldwide each year.
When these perforations do not heal on their own, they require reconstructive “tympanoplasty” surgery to repair the defects using graft materials taken from other parts of the body, such as the fascia. However, these grafts do not always ensure full mending of the eardrum, meaning that patients often require revision surgeries and could suffer long-term hearing loss.
In response to the need for a better and longer-lasting eardrum reconstruction method, Black began developing the PhonoGraft six years ago, working with Dr. Aaron Remenschneider at the Massachusetts Eye and Ear hospital in Boston, and Professor Jennifer Lewis at Harvard University.
The PhonoGraft is a biomimetic graft 3D printed from biodegradable elastomers that functions like a native eardrum, and due to the use of 3D printing can accommodate different ear sizes and requirements. Once inserted into the patient’s ear, the Phonograft effectively conducts sound and promotes native tissue and vascular cell growth while slowly biodegrading. As a result, new eardrum tissue is regenerated inside the ear that is capable of conducting sound and providing normal barrier functions against pathogens.
“PhonoGraft promotes tympanic membrane regeneration by combining biodegradability with its biomimetic architecture composed of circular and radial features,” said Black. “This graft conducts sound well at both low and high frequencies, thus restoring function far better than autologous tissue grafts, which are currently used.
“Moreover, we expect that the PhonoGraft will improve patient healing and hearing outcomes, thereby reducing the need for revision surgeries.”
The road to FDA approval
Black and her team have previously validated the Phonograft in preclinical in-vivo studies using a chinchilla model with an eardrum size and hearing range similar to that of humans. They observed the PhonoGraft facilitate the remodeling of native tissue in animals with chronic perforations while it slowly degraded, and carried out auditory tests that determined the 3D printed device restored hearing “far more effectively” than traditionally-used autologous tissue grafts.
Now, Black is seeking 510K FDA clearance for the PhonoGraft, which she told the Detriot Free Press could potentially enable doctors to carry out “dozens of eardrum repairs throughout the day”. She is also reportedly working on designs that could allow the Phonograft to be placed in the ear canal of patients while they are awake, requiring only local anesthetic as opposed to undergoing more time-consuming and expensive general anesthesia.
Going forwards, once FDA clearance is received Black hopes the PhonoGraft will be marketable to ear, nose, and throat surgeons by the end of next year, or early 2023.

3D printing in regenerative medicine
3D printed biostructures for use within regenerative medicine and tissue engineering have received increasing attention in recent years, with new bioinks being continually developed that are cable of supporting long-term cell growth and survival.
In the past year alone, 3D printing has played a part in developing biocompatible constructs that mimic human-sized airways able to support new cell and blood vessel growth, and in rapidly speeding up the process of fabricating cell-laden hydrogel structures that could one day lead to 3D printed human tissue transplants.
Elsewhere, the technology has enabled the simultaneous printing of skin and bone tissue to repair injuries, and has also moved scientists one step closer to being able to regrow teeth from the root.
Aside from research breakthroughs, the likes of 3D printer OEM 3D Systems and 3D bioprinter manufacturer CELLINK have also made strides in the regenerative medicine sphere. After announcing plans to scale up its bioprinting activities in light of the progress seen with its Print to Perfusion platform earlier this year, 3D Systems recently unveiled a co-development agreement with regenerative medicine firm CollPlant to develop 3D bioprinted soft tissue structures for breast reconstruction treatments.
CELLINK, meanwhile, acquired in-vitro technology specialist MatTek Corporation in March to advance its research into animal cruelty-free cellular testing models, and a couple of months later acquired Two-Photon Polymerization (2PP) 3D printer manufacturer Nanoscribe with plans to use the firm’s technology to fabricate more lifelike soft tissues.
Representatives from both 3D Systems and CELLINK took part in 3D Printing Industry’s recent webinar on Regenerative Medicine and 3D Printing: Opportunities, Challenges, and Future Direction, which is now available to watch on our YouTube Channel.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows the 3D printed PhonoGraft biomimetic graft. Photo via Wyss Institute.



