3D printed Cellular Titanium® spinal support implants are now approved by the U.S. Food and Drug Administration (FDA).
Made by German medical device firm Emerging Implant Technologies GmbH (EIT), the cages have shown an impressive turnaround from production to market, helping to validate 3D printing’s place as a viable method of fabrication in medicine.

Worldwide recognition
To date, Cellular Titanium implants developed by EIT have been used in 10,000 cases in over 15 countries around the world. 510(k) clearance from the FDA allows EIT to bring the product to market in the U.S for the first time.
“This is a major milestone for EIT.” says Guntmar Eisen, company Co-Founder and CEO, “We look forward to bringing our unique technologies to the United States and partnering with top tier surgeons and institutions to bring the best results to patients that are in need of these devices.”
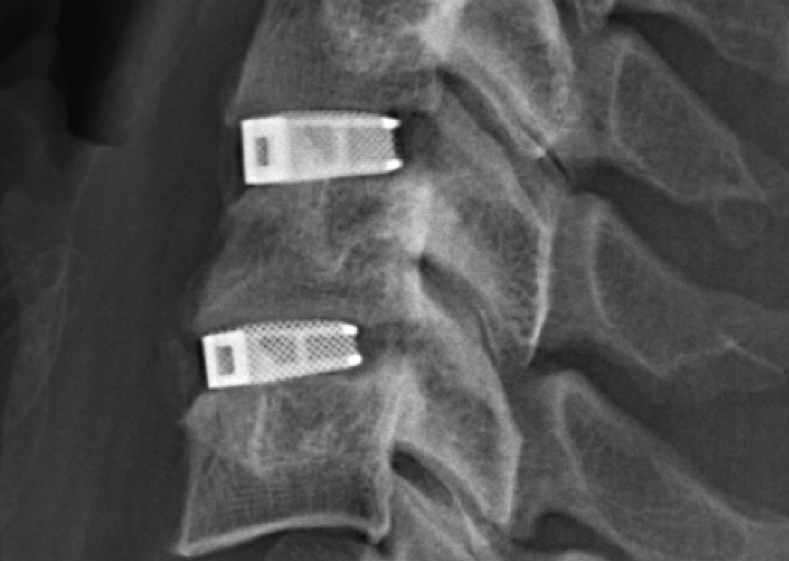
Super spinal support
EIT Cellular Titanium implants are made using the Selective Laser Melting (SLM) additive manufacturing technique.
Producing the devices in this manner allows EIT to achieve 80% porosity of the parts, making them conducive to cell growth essential for bone repair.
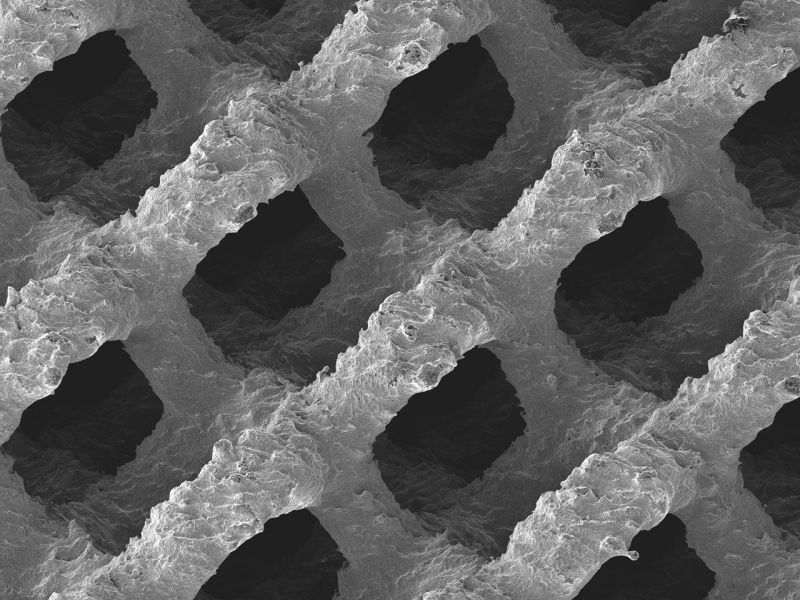
Furthermore, if research uncovers new parameters for optimal pore or implant design, SLM will allow EIT to easily re-design the devices and re-apply for approval.
Competition spikes
By entering the market at this time, EIT joins a rapid flux of competition.
Virginia medical device manufacturer K2M (NASDAQ:KTWO) recently achieved FDA approval for its adjustable, 3D printed MOJAVE PL lumbar support cage.
As of March 2016, Stryker’s 3D printed Tritanium lumbar cage also has FDA approval, and has been moving to introduce 3D design into the production of implants made from PEEK plastic.
For all the latest 3D printing news, subscribe to the most widely read newsletter in the 3D printing industry, follow us on twitter and like us on Facebook.
Featured image: Animation demonstrating the probity of EIT’s 3D prints implants. Screenshot via EIT Emerging Implant Technologies



