Rounding off another tumultuous year, December was certainly not short of news. In addition to multiple medical advances, the month saw court injunctions handed out, and the legalization of a controversial suicide capsule.
Read on for the standout developments in December, which involved the likes of Desktop Metal, Essentium, Redefine Meat, Prodways, CELLINK, and others.
Click to see all articles in our 2021 3D Printing Industry Review of the Year series.
Remembering E3D Founder Sanjay Mortimer
First, though, December began with the devastating news that E3D Founder Sanjay Mortimer had sadly passed away. A well-loved and inspirational leader, Mortimer spent the past decade tirelessly committed to his vision of changing the way humanity manufactures goods, having helped to create a community that strives to advance 3D printing and knowledge-sharing.
Once again, the 3D Printing Industry team sends our heartfelt sympathy to Mortimer’s family, friends and colleagues. E3D has since set up a discord channel where you can share your memories and tributes.

EOS installs 1000th printer
Powder bed fusion 3D printer provider EOS delivered and installed its 1000th 3D printer in North America in December. The AMCM M 4K machine was purchased by Florida-based 3D printing service provider Sintavia, bringing its total number of EOS 3D printers to 17.
“Constant innovation, consistency between machines, and industrial-scale production dependability are why aerospace manufacturers of tomorrow rely on EOS,” said Brian Neff, CEO of Sintavia.
“Their machines form the foundation of our company’s manufacturing technology, and we are thrilled to be part of this milestone achievement in EOS’ history.”
Another month, another SPAC
December also saw industrial 3D printer manufacturer Essentium become the latest to join in the SPAC (Special Purpose Acquisition Company) boom that has taken hold of the 3D printing industry this year. Essentium plans to go public on the Nasdaq under the ticker ‘ADTV’ by merging with SPAC Atlantic Coastal Acquisition.
Expected to be completed by the end of Q1 2022, the deal will see the firms combine into a joint venture worth an estimated $974 million, and will raise a projected $346 million to fund the venture’s organic growth.
“We believe that following this transaction, Essentium will be extremely well-positioned for rapid growth as it further expands its ecosystem offerings, capitalizes on its line-of-sight sales pipeline, and executes on its M&A strategy as it continues to advance AM as a public company,” said Tony Eisenberg, CSO of Atlantic Coastal Acquisition.
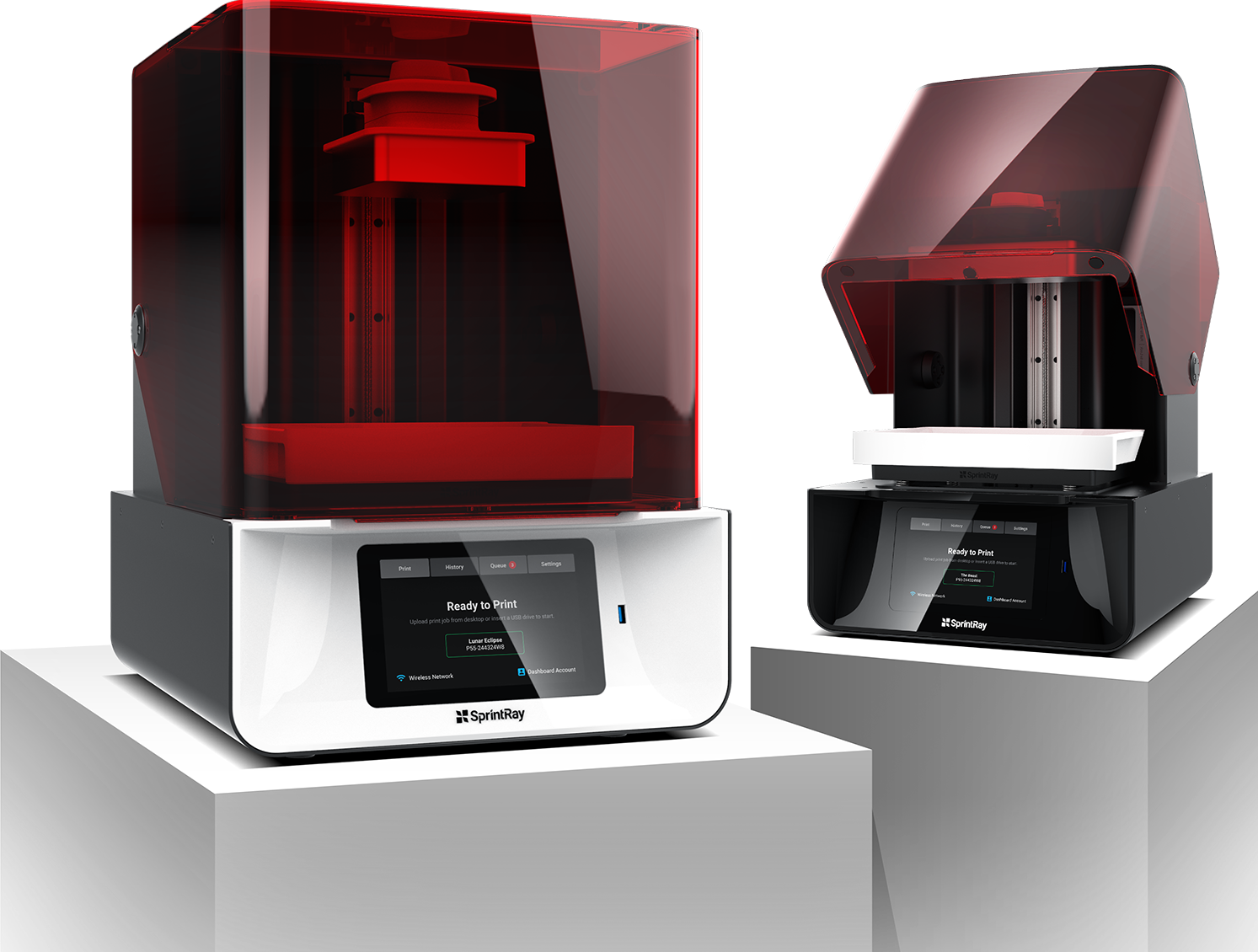
Desktop Metal vs SprintRay
Meanwhile, industrial 3D printer manufacturer Desktop Metal was granted a preliminary injunction by a court in Germany that prevents SprintRay from selling its dental systems there. Desktop Metal alleges that the technology leveraged by SprintRay’s Pro 95 and Pro 55 machines infringes upon patents covering the ‘layer separation process’ of its subsidiary EnvisionTEC.
As a result of the ruling, SprintRay is now prohibited from selling, importing, using, or storing any product in Germany that is found to violate these patents. SprintRay is yet to comment publicly on the ruling.
3D printed ‘meat’ wins Marco Pierre White’s seal of approval
Redefine Meat, a food 3D printing firm developing animal-free meat, revealed it had received glowing praise from top European chefs for its New-Meat range of plant-based 3D printed meat products, made commercially available to high-end restaurants in December.
The roll-out reportedly marks the first time high-end restaurants will offer plant-based whole cuts as part of their menus, backed by leading chefs including British chef and restaurateur Marco Pierre White and Michelin-starred Dutch chef Ron Blaauw.
Pierre White said he was “mind-blown” by the company’s New-Meat offering, adding: “Redefine Meat’s New-Meat products are pure genius, giving you all the sustainability and health benefits of plant-based, without the compromise on taste and texture.”

Controversial suicide capsule legalized
December also saw a 3D printed pod that allows users to climb inside and take their own lives given legal clearance in Switzerland. Named Sarcophagus, or ‘Sarco’ for short, the controversial euthanasia system is made up of a coffin-like 3D printed capsule mounted onto a stand that releases nitrogen and allows for suicide via inert gas asphyxiation.
Having been around since 2018, the device’s creator Dr. Philip Nitschke, nicknamed “Dr Death” by some of his critics, now says there are “no legal issues at all” with putting it into practice from as soon as next year.

3D printed battery production begins
Elsewhere, Blackstone Resources, a Swiss investment firm focused on battery technology, announced series production of its 3D printed battery cells had commenced at its manufacturing plant in Döbeln, Germany. Having spent the last two years developing and refining its lithium-ion battery 3D printing technology, the company has now launched its first large-scale production facility and expects to reach its 500MWh annual production capacity target by the end of next year.
Going forwards, the firm’s long-term goal is to produce battery cells every second, starting with the expansion of its team from 14 to 38 employees in the next year.
“The vision – with a single machine park, we print cells in different shapes, different electrodes, and electrolyte materials quickly and cost-effectively on a large scale,” said Holger Gritzka, Managing Director of Blackstone Technology, the firm’s research, and development subsidiary.

Advances in medical 3D printing
December 2021 saw a multitude of 3D printing-related medical announcements and developments make the headlines.
First up, pharmaceutical 3D printing specialist FabRx, University College London, and Universidade de Santiago de Compostela (USC) developed a novel 3D printed device capable of removing drugs from water. The device could help to address the global issue of infiltration of pharmaceutical drugs into environmental water supplies, which can have huge impacts on both ecological equilibrium and human health.
Meanwhile, Prodways was awarded a contract that would see its machines produce up to a million dental aligners per year. The ‘major industrial project’ is part of an initiative by a ‘world-leading medical and dental distributor’, for which Prodways will ship eight of its MOVINGLight 3D printers and related materials to multiple sites across the US.
Over the next 18 months, the installation base could grow to as many as 20 machines, providing Prodways with its largest project to date and a recurring source of revenue for years to come.
Elsewhere, December saw Health Canada approve its first Canadian-made 3D printed medical implant, a customizable mandibular plate for use in facial reconstruction surgery. The implant was developed by the 3D Anatomical Construction Laboratory (LARA 3D), which received ISO 13485 certification in April, and is called the Specifit 3D mandibular plate. The approval enables surgeons to use the implant, together with two surgical cutting and drilling guides, to treat patients with greater customization.

Moving onto hardware, 3D printer manufacturers CELLINK and Nanoscribe, which are both sister companies under the BICO banner, announced their new jointly-developed Quantum X Bio 3D printing system in December. The machine combines CELLINK’s bioprinting capabilities with Nanoscribe’s Two-Photon Polymerization (2PP) technology to deliver the “world’s most accurate 3D bioprinter”, according to the firms.
Designed to miniaturize bioprinting, the Quantum X Bio is aimed at research institutions and R&D teams interested in advanced biomedical applications like tissue engineering, mechanobiology, and regenerative medicine.

Another “world-first” claim came from South Korean-based photopolymer resin manufacturer Graphy, who unveiled what it says is the first direct 3D printed aligner that features a shape memory function. The aligner is reportedly the only one currently on the market that has this feature, which is slated to accelerate the treatment process for patients and remove the need for replacement aligners.
The aligner is 3D printed with Graphy’s Tera Harz photocurable resin, which is engineered with two carbon backbones to allow the material’s molecular formula to be closely controlled and customized to specific customer requirements. If deformed or stretched, the aligner’s shape can be restored when it comes into contact with hot water.
Finally, December saw the US Food and Drug Adminstration (FDA) issue a public call for comment on a future-proof regulatory framework it is devising to ensure the quality of 3D printed medical devices. The FDA document outlines how point-of-care 3D printing is governed, identified challenges to its end-use, and offers a revised potential ruleset. With the release of the paper, the FDA is also requesting feedback from the medical 3D printing industry that will help to inform future regulation.
“The 3D printing of medical devices is at the forefront of innovation and health care,” said William Maisel and Ed Margerrison of the FDA’s OSEL and CDRH divisions.
“The discussion paper we’re sharing today provides insight into our perspective of the benefits and challenges of 3D printing at hospitals and other points of care, and presents a potential approach for regulatory oversight.”
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows Sanjay Mortimer. Photo via E3D.



