Chicago-based biofabrication firm Dimension Inx has announced that CMFlex, its FDA-approved 3D printed regenerative bone graft product, has been used in its first two clinical cases.
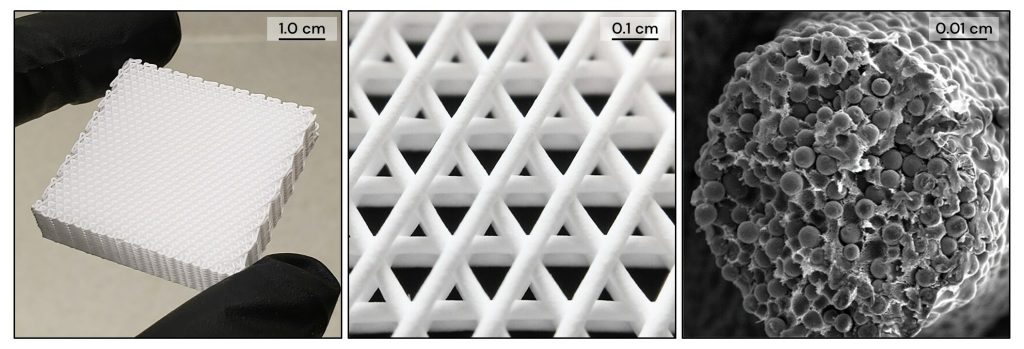
The 3D printed synthetic bone graft is targeted towards the treatment of various bony defects in oral and maxillofacial surgical applications. CMFlex’s three-dimensional architecture incorporates macro, micro, and nano-level feature dimensions which promote bone regeneration.
These first two surgical procedures included a mandibular angle augmentation (surgery of the lower jaw) and a maxillary segmental osteotomy (surgery of the upper jaw). The two cases were carried out by Dr Derek Steinbacher, Director of West River Surgery Center, and Dr Brian Farrell, DDS, MD, of the Carolinas Center for Oral & Facial Surgery.
“These first cases are not only indicative of a new generation of biomaterials but also highlight our technology platform’s unique capability to rapidly create biomaterials that direct cell behavior to restore tissue and organ function,” commented Dr Adam Jakus, CTO and Head of Technology Strategy at Dimension Inx. “It is a proud moment for us to be able to demonstrate the value of therapeutics derived from integrating novel biomaterial design and 3D-printing approaches.”
“We are thrilled to share this solution with the surgical community. CMFlex is a product that represents our unique approach to restoring functionality in the body: it’s a dynamic collaboration between biology, material composition, microstructure, and macroarchitecture,” added Dimension Inx’s CSO and Head of R&D, Dr Ramille Shah.
“We’re excited by the interest we’ve already received from surgeons who recognize the importance of a ready-to-use solution with great handling characteristics and the ability to cut and shape the graft to match the defect site.”
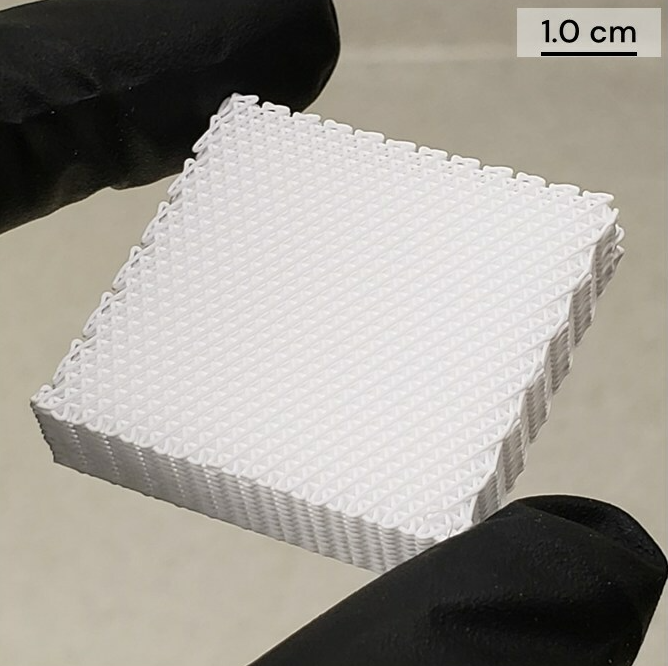
CMFlex: a 3D printed regenerative bone graft
CMFlex became the first 3D printed regenerative bone graft product to acquire FDA clearance last year. Dimension Inx’s product is currently available to a limited number of surgeons, with a broader release expected in 2024.
CMFlex’s chemistry primarily comprises a combination of hydroxyapatite particles and biodegradable polylactide-glycolide (PLG) polymer. Both of these materials possess a reputation for demonstrating biocompatibility and clinical utility.
These base materials are combined into a proprietary, microstructurally porous composite material called Hyperelastic Bone, which is then 3D printed into CMFlex. According to Dimension Inx, the resulting product can be sized by the surgeon to meet the specific needs of a patient. CMFlex is also said to effectively absorb fluids, enabling it to control bleeding during surgery while assisting the bone remodeling process once implanted.
“CMFlex offers a novel ready-to-use bone graft solution that boasts excellent handling characteristics, and the fact it can also be cut and trimmed to easily match the defect site made it an exceptional product to work with”, explained Dr Steinbacher. Dr Farrell added that “the material was easy to contour, and I anticipate ongoing utilization to monitor its performance over time”.
In addition to these two regenerative procedures, CMFlex has been used in several socket preservation surgeries for future dental implant placement. “I have been thoroughly impressed with the product’s ability to promote hemostasis, which simplified the implantation process”, stated Dr Robert Bosack, DDS, Oral, Maxillofacial & Dental Implant Surgery.
Advancements in medical 3D printing
3D printing is an ever growing technology within the medical industry, especially with regard to bone-related procedures.
Earlier this year, it was announced that RWTH Aachen University’s Digital Additive Production facility (DAP) is researching a novel zinc-magnesium alloy combination for lattice structures as part of the BioStruct project. Produced using Laser Beam Powder Bed Fusion (PBF-LB), the BioStruct consortium is working to optimize these 3D printed bioabsorbable implants for patient-friendly bone healing.
Elsewhere, a 3D printed bone graft from medical device manufacturer Cerhum was given approval for use in patients throughout Europe last year. Called MyBone, this offering was reportedly the first commercially available 3D printed bone graft under the Medical Device Regulation 2017/745, and also received ISO 13485 certification. The patient-specific bone graft has since been made available to maxillofacial and orthopedic surgeons throughout the continent.
Subscribe to the 3D Printing Industry newsletter to keep up to date with the latest 3D printing news. You can also follow us on Twitter, like our Facebook page, and subscribe to the 3D Printing Industry Youtube channel to access more exclusive content.
Are you interested in working in the additive manufacturing industry? Visit 3D Printing Jobs to view a selection of available roles and kickstart your career.
Featured image shows CMFlex’s three-dimensional architecture. Image via Dimension Inx.


