UK-based medical 3D printing specialist 3D LifePrints announced its rebranding as Insight Surgery, at the official launch of its US business in Houston.
The rebranding coincides with the company receiving its second FDA 510(k) clearance for non-joint replacing osteotomies in the appendicular skeleton including bone tumor resection. 3D LifePrints claims, it is thought to be the widest clearance obtained thus far, encompassing both the upper and lower extremities. In addition, Insight Surgery revealed the opening of its cleanroom production facility at the Texas Medical Center. According to 3D LifePrints, the rebranding represents the organization’s progression from a UK-based medical 3D printing business to a cross-Atlantic provider of Personalized Surgery.
Paul Fotheringham, Insight Surgery CTO said, “Our 2 recent FDA clearances enable us to bring our full range of CMF and Orthopaedic devices to the US market. Our aim is to put these highly impactful devices in surgeons’ hands within 5 days of receipt of scan data.”
3D LifePrints to Insight Surgery – a sophisticated journey
The official USA launch of Insight Surgery took place at the residence of the British Consulate General in Houston. It was attended by many members of Houston’s medical and academic communities, along with representatives from Houston Methodist, Texas Children’s Hospital, MD Anderson, and Texas Medical Center Innovations.
“I’m delighted to be back again amongst so many friends and colleagues in Houston. Texas quickly became our first choice as a landing spot and given its proximity to so many world-class hospitals, the Texas Medical Center is an ideal location for our first US facility,” added Peter Ellingworth, Chairman of Insight Surgery.
The FDA-cleared digital platform EmbedMed from Insight Surgery is an end-to-end solution for surgeons that digitizes the surgical planning procedure and allows for the quick design and manufacture of patient-specific medical devices. Embedmed’s capabilities entail patient scan data processing and serving as a pre-operative software tool for simulating and assessing surgical planning options. The system’s output files can be delivered in digital format or as surgical guides and physical models to be employed during surgery.
“Firstly, I’d like to thank Houston for such a warm welcome. We have chosen the launch of our US business as the ideal time to rename the company to “Insight Surgery” so that it aligns with our focus on Digital Planning and Personalized Surgery. Having supplied Personalized Devices to over 1000 surgeries in the UK we now turn our attention to the US market, specifically Texas,” said Henry Pinchbeck, Co-Founder, and Chief Executive Officer.

AM companies getting a fresh start with rebranding
Previously, the parent company of 3D bioprinting firm CELLINK rebranded itself ‘BICO’ and disclosed that its net sales increased by more than 600% during the first half of 2021. As per BICO’s financials, the company earned $48 million in H1 2021, $39.1 million more than it did in the same period in 2020. BICO highlighted its increased revenue to “positive demand” from its repeating clientele, in addition to the larger install base gained through its acquisition-led growth strategy, which resulted in consumable sales accounting for 23% of total revenue in Q2 2021.
Moreover, Sakuu, which rebranded from KeraCel in 2021, has focused on 3D printing solid-state batteries ever since for uses like electric cars. The Sakuu AM Platform, developed by the company, integrates powder bed technologies with material jetting and is compatible with ceramics, metals, and the company’s proprietary ‘Poralyte’ support material. According to the company, 3D printing technology can produce solid-state batteries that are half the size and double the capacity of conventional lithium-ion cells, while also being “much less” expensive in large quantities. Sakuu also received three patents, each of which was intended to enhance the effectiveness and adaptability of the company’s multi-material additive manufacturing capabilities.
What does the future of 3D printing for the next ten years hold?
What engineering challenges will need to be tackled in the additive manufacturing sector in the coming decade?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
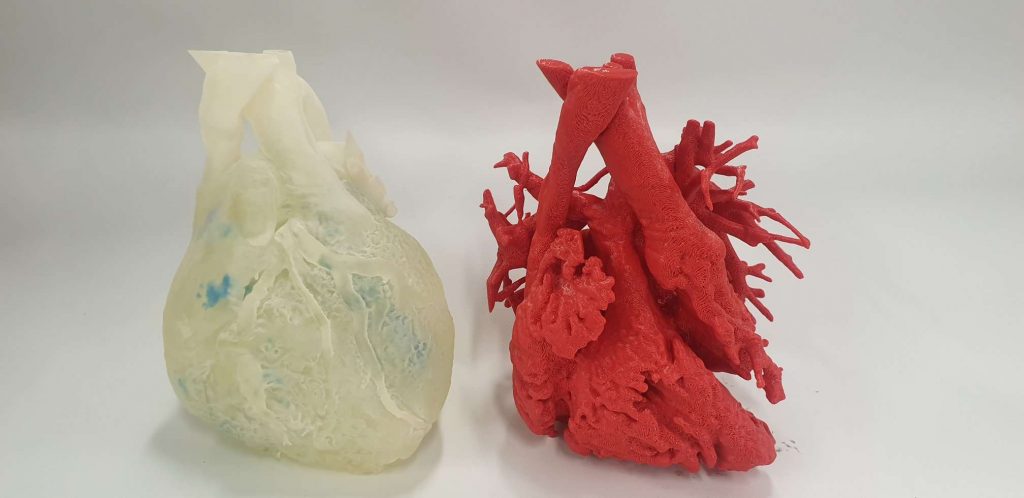
Featured image shows 3D Lifeprints technology has been utilized in a range of medical applications including soft printed models to simulate operations (pictured). Photo via 3D Lifeprint.



