In response to the Covid-19 pandemic, the 3D printing industry has successfully plugged many of the supply chain gaps in the medical sector resulting from border closures, increased demand, and workforce shortages.
To make sure these products adhere to the strict quality requirements and legal regulations medical devices are subject to, technical service giant TÜV SÜD has pulled together several checklists for 3D printing processes and provided them to manufacturers throughout the pandemic.
“When borders are closed to stop the spread of Covid-19, companies are forced to adjust their supply chains,” said Gregor Reischle, head of additive manufacturing at TÜV SÜD.

in Munich, Germany. Image via TÜV SÜD.
3D printing during the pandemic
Since the beginning of the coronavirus pandemic, additive manufacturing sites with 3D printer access have reacted to the need to fill in gaps that had appeared in the supply chain, re-purposing their production processes to meet the demand for nasal swabs, ventilator components, and PPE, in addition to many other medical components.
3DPI has been tracking how the 3D printing industry has responded to the Covid-19 pandemic since April, detailing the projects and initiatives set up by professional AM providers, makers, and designers in the 3D printing community.
The likes of Photocentric, which landed a contract by the UK government to manufacture over 7.6 million 3D printed protective face shields over the next few months, and the University of Sheffield, which also received government funding to scale up its delivery of PPE to a refugee camp in Jordan, have been busy plugging the global PPE shortage.
Elsewhere, students from Massachusetts-based Smith College have produced a novel “Smithvent” partially-3D printed ventilator, while Helpful Engineering volunteers developed a 3D printed timing mechanism of an emergency ventilator based on a U.S. Army design from 1965. Most recently, global reinsurance business Munich Re and technology research firm Fraunhofer awarded €1m as part of a competition to 3D print medical devices for COVID-19 sufferers.
According to TÜV SÜD, even before the pandemic arose, additive manufacturing in the medical sector had been forecast by analysts to be worth some $20 billion. Presently, 3D printing is utilized in this sector for a multitude of applications spanning general healthcare to high-precision personalized devices.

The checklists
Before they make it to market, 3D printed medical devices must prove they conform to a range of safety, quality, and performance standards. Conformity assessments take time, and so TÜV SÜD developed several checklists to guide manufacturers in implementing these requirements speedily and reliably.
The checklists set out the main requirements facing additive manufacturing for the medical sector in relation to key standards and regulations. Beneficial for testing laboratories, healthcare specialists, and the public, the lists have been supplied free of charge throughout the pandemic.
International standards organizations ASTM International and ISO have also provided free access to relevant standards for PPE and medical devices.
In addition to playing an important role during the pandemic, believes additive manufacturing is having a positive impact on the medical and healthcare sector more generally, too. “There are many indications that fast, integrated supply chain networks with local production operations will become the new normal,” he said.
In addition to providing checklists, TÜV SÜD also provides specific tests for additive manufacturing processes, enabling 3D printing companies to verify their conformity with certain requirements.

Collaborating with industry
TÜV SÜD has also partnered with several 3D printing firms, organizations, and authorities to help combat the effects of the coronavirus pandemic.
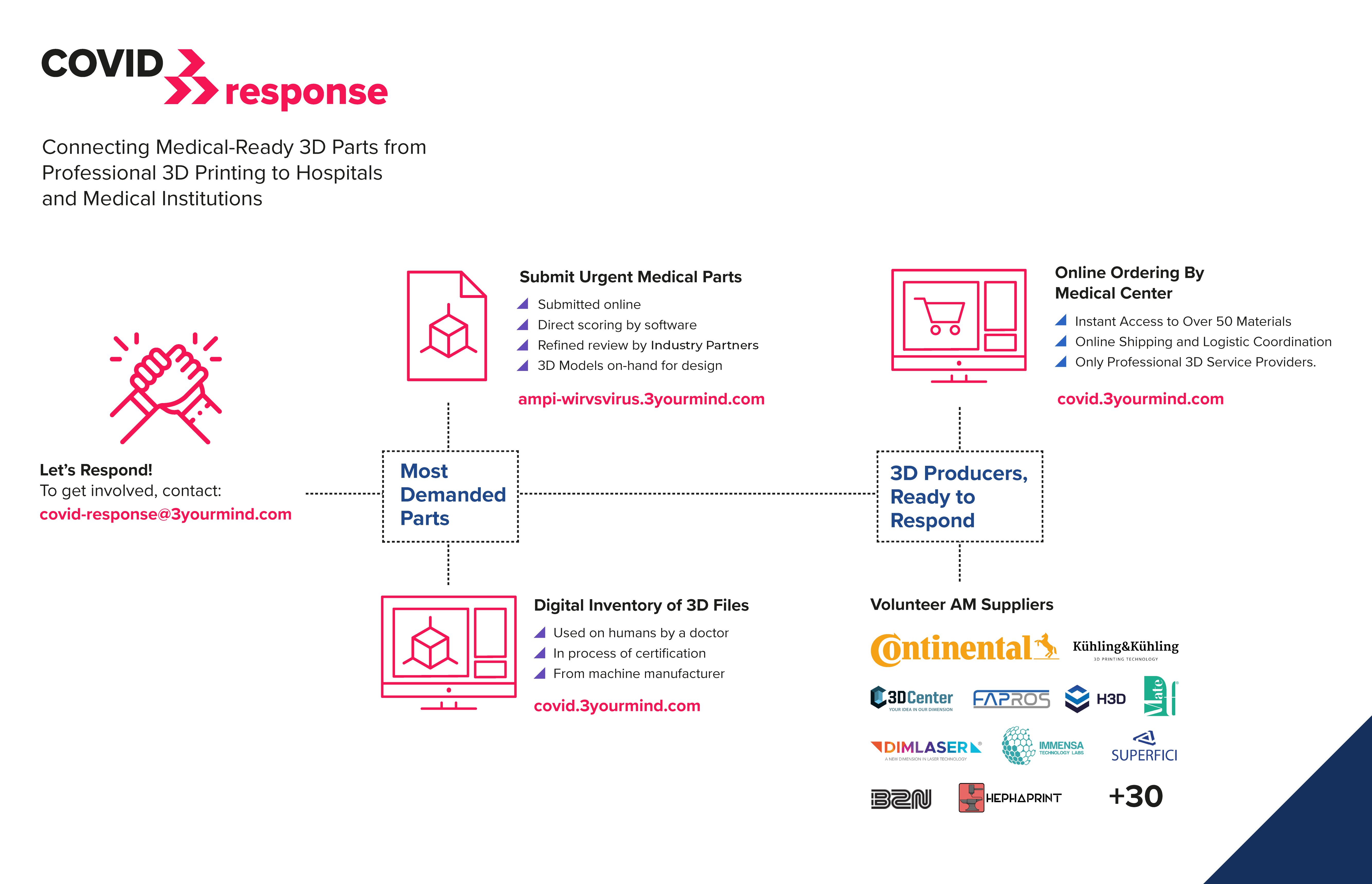
Production software provider 3YourMind embedded TÜV SÜD’s checklist in its workflow after creating a platform to coordinate and organize supplies of essential products throughout the pandemic.
TÜV SÜD also worked with 3D printer manufacturer Ultimaker on its first checklist, in addition to participating in an inter-agency collaboration between the Nanyang Technological University (NTU) and Singapore’s National Additive Manufacturing Innovation Cluster (NAMIC) to guide manufacturers through relevant testing requirements.
The technical firm is a member of the user-oriented network Mobility goes Additive (MGA) which is building a database of use cases and FAQs on 3D printing face visors, masks, and ventilators. TÜV SÜD is supporting member manufacturers in implementing regulatory requirements.
Nominations for the 2020 3D Printing Industry Awards are still open, let us know who is leading the industry now.
The fourth edition of the 3D Printing Industry Awards Trophy Design Competition is now underway. Enter your design for the chance to win a CraftBot Flow 3D printer.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows TÜV SÜD conference hall. Photo by Kubi Sertoglu.



