SME, an organization working closely with manufacturing professionals, companies and educators to generate solutions to manufacturing industry challenges, has announced the release of the Medical AM3DP 2018 Annual Report at SME’s RAPID + TCT event in Fort Worth. The report provides an overview of the impact of additive manufacturing/3D Printing (AM3DP) in the medical field, underlining the importance of technological innovation to improve cost-effective patient care.
Survey indicates 3D printing is on the rise
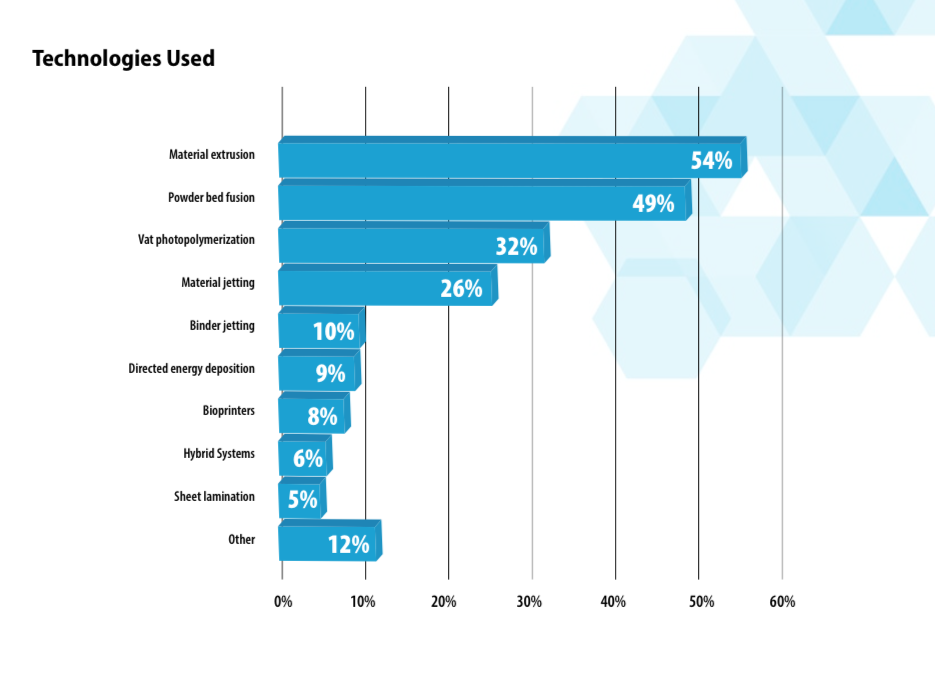
The report includes the findings of a survey of medical AM3DP professionals to provide a insights into how additive manufacturing in the healthcare sector is being harnessed, which processes and materials are being used, what challenges arise, and what is expected to impact patients next.
The study finds 11% of industry revenues were derived from Medical/dental pieces, i.e. 3D printed implants, medical devices and other components for healthcare.
Healthcare is set to continue as a leading practical use of 3D printing with 97% of AM professionals confident of an increase in Medical AM/3DP applications.
The SME Medical AM3DP workgroup also identified several developments, from collaborations to improve workflows to growth in future technologies and applications.
Device Manufacturers Help Transform Healthcare Delivery
According to Dan Fritzinger, Manager at Global Instrument Innovation for DePuy Synthes, part of the Johnson & Johnson family of companies, the use of medical 3D printing technology has the potential to transform healthcare delivery to reach personalized healthcare solutions for patients and consumers. He states:
“3D printing was once an innovation of the future and is now an exciting reality. This technology presents enhanced career growth opportunities for each new generation of engineers and manufacturers entering the workforce.”
Lauralyn McDaniel, Industry Manager at SME, said:
“Collaboration between companies and organizations, and particularly between medicine and engineering, was a big part of the story this year. Everyone is working together to continue to grow the millions of patients already directly impacted by the benefits of AM/3DP-enabled precision medicine.”
Activities from regulatory agencies, industry and clinical groups, and technology providers are helping to expand the impact of medical 3D printing which already extends to millions of patents. These groups include the U.S. Food and Drug Administration (FDA), SME Medical Additive Manufacturing/3D Printing Workgroup, RSNA 3D Printing Special Interest Group, DICOM Workgroup-17 3D Manufacturing, and Additive Manufacturing Standardization Collaborative (AMSC).
You can read more of our coverage from Texas at this weeks RAPID + TCT 2018. New printers included the Arcam EBM Spectra, GE Additive’s new additive manufacturing system, Aleph Objects’ LulzBot Mini 2 3D printer, and new metal 3D printers presented this year.
For all the latest 3D printing news – subscribe to the 3D Printing Industry newsletter, follow us on Twitter, and like us on Facebook. Follow the Awards with the hashtag #3DPIAwards.
The 3D Printing Industry Jobs is live. Post a job or discover your next career move now.
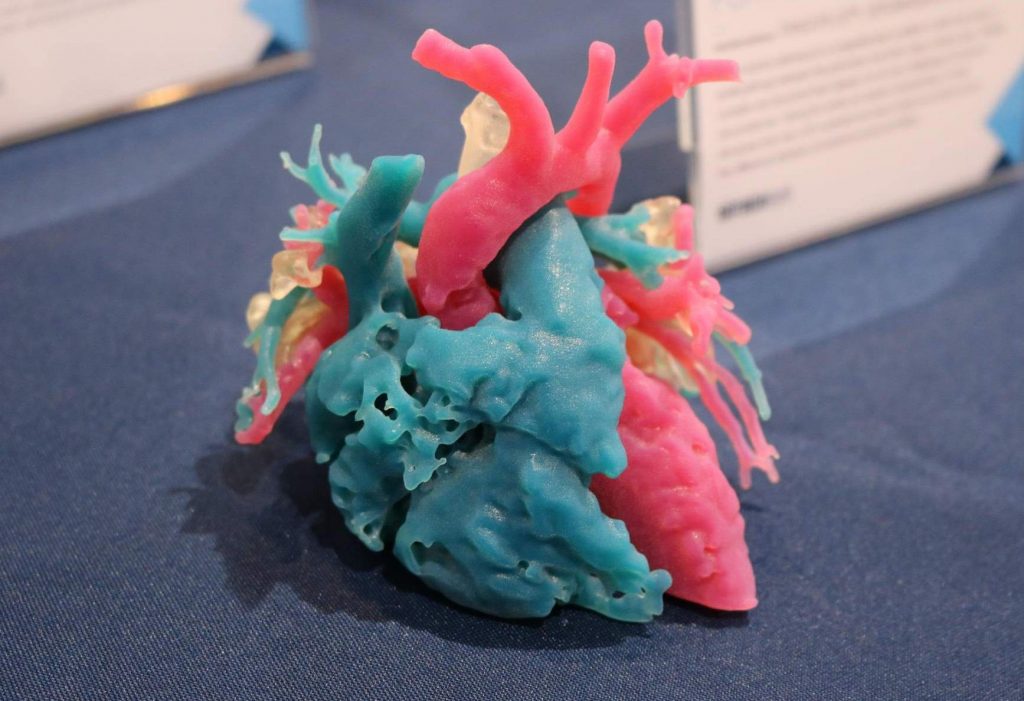
Featured image shows surgeon holding 3D printed medical device. Image via cmfenews


