UK-based engineering technologies company, Renishaw, has announced a milestone in a medical study seeking to help sufferers with progressive nervous system disorder Parkinson disease. The study looks at a patented intraparenchymal drug delivery device and is the completion of a joint Phase 1-2 clinical study with pharmaceuticals expert Herantis Pharma.
The device played a critical role in the investigation of cerebral dopamine neurotrophic factor (CDNF) as a treatment for Parkinson’s disease.
Initial results are promising, as they indicate predictable and accurate placement of the device as well as significant efficacy and safety of both the device and CDNF.
Parkinson’s disease
Parkinson’s disease is a neurodegenerative disease, caused by the progressive break-down of neurons responsible for the synthesis of dopamine in the brain. Primary symptoms include involuntary shaking, muscle stiffness, and the slow down of movement. Non-motor symptoms include sleeping difficulties, memory loss, and degeneration in mental health.
These symptoms can be managed initially with medication but there is currently no long-term treatment that prevents the progression of the disease.
The intermittent drug delivery system
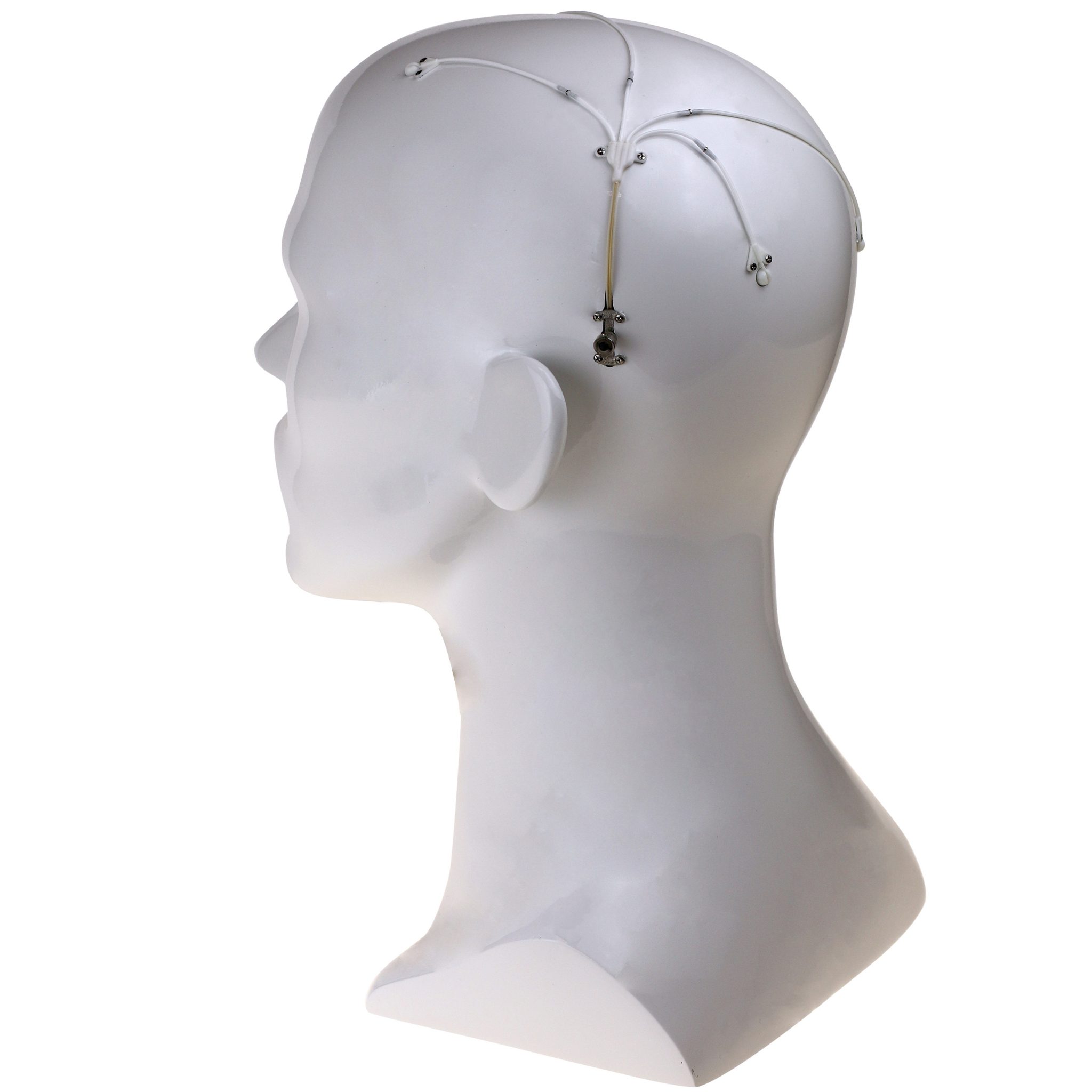
Renishaw’s award-winning device is comprised of up to four catheters which are implanted into relevant areas in the brain. 3D printed titanium transcutaneous ports are implanted behind the patient’s ear, allowing medical professionals to access the catheters. Drug-filled infusion lines are then connected using MRI-compatible components and retractable needles are extended through a septum to allow therapeutics in the external infusion lines to be infused through the catheters.
The patented design allows patients to receive medication in an out-patient setting, where new catheters do not need to be re-implanted with every infusion.
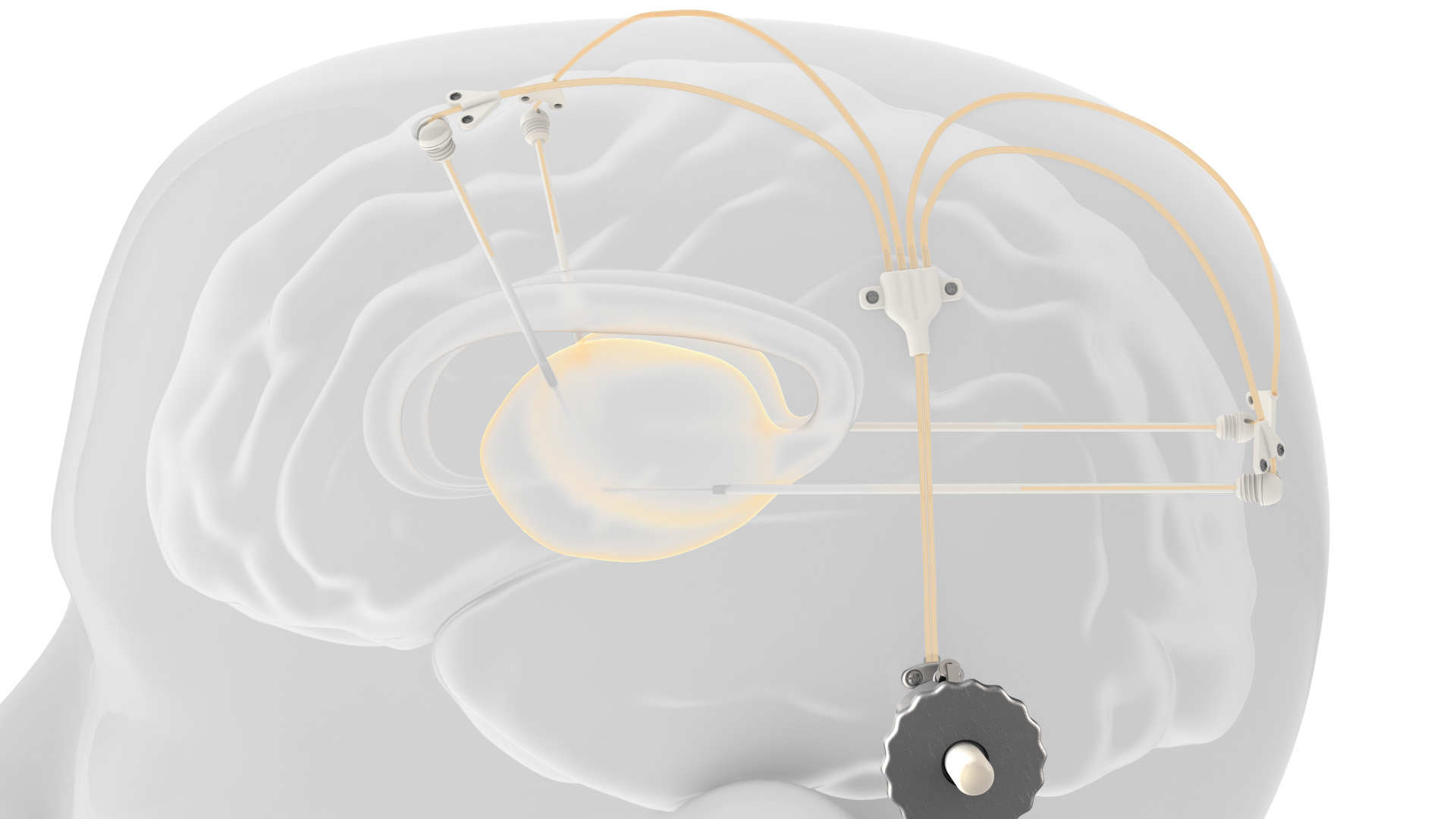
The study
The study was the first of its kind to be trialed with humans, where 17 patients were randomized to either receive placebos for six months or one dose of CDNF every month for six months. After the six months, patients may enter into a secondary study where everyone is given CDNF with Renishaw’s device.
Researchers evaluated the safety and performance of both the device and CDNF. This was done by rating the patients against the United Parkinson’s Disease Rating Scale (UPDRS) motor score evaluation. The long-term nature of the study allows for a prolonged therapeutic window. This is crucial in assessing the neuroprotective and neurorestorative capabilities of CDNF.
While initial results are promising, researchers will continue to assess the data and add to it the results of the secondary study to form a more complete conclusion.
Rupert Jones, Managing Director of Renishaw Medical, stated: “The results of this trial and the performance of Renishaw’s drug delivery system are promising for the many people with Parkinson’s disease and I would like to take this opportunity to thank the trial participants for making this possible.”
He added, “These results allow us to build towards CE marking of Renishaw’s device so that further neurodegenerative and neuro-oncological conditions can benefit from our technology. We see our device as an enabling technology that facilitates the reliable and repeated delivery of therapeutic agents directly to targets deep within the parenchyma, as part of a paradigm shift in the way treatments of neurological disorders and brain tumours are progressing.”
The study received funding from the European Union’s research and innovation program Horizon 2020.
This is not the first time 3D printing technology has been used in medical implants. In South Africa, surgeons completed a landmark ear operation using 3D printed titanium to reconstruct parts of an ear. Elsewhere, in Germany, clinical trials focused on 3D printed breast implants have commenced.
The nominations for the 2020 3D Printing Industry Awards are now open. Who do you think should make the shortlists for this year’s show? Have your say now.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows Renishaw’s drug delivery device. Image via Renishaw.



