Texan medical device company Osseus Fusion Systems, has received FDA clearance for its family of 3D printed spinal implants known as Aries.
The implants are designed to help surgeons ease back pain and shorten spinal recovery time. They are the first example of Osseus’ new line of 3D printed products, with several other 3D printed medical devices planned for the near future.

Improving spinal implants
Founded in 2012 by Eric Hansen and Robert Pace, Osseus Fusion Systems focuses on the development of advanced medical products for spinal related injuries. One of Osseus’ key goals is using new, emerging technologies such as additive manufacturing, to improve the quality and capabilities of its products.
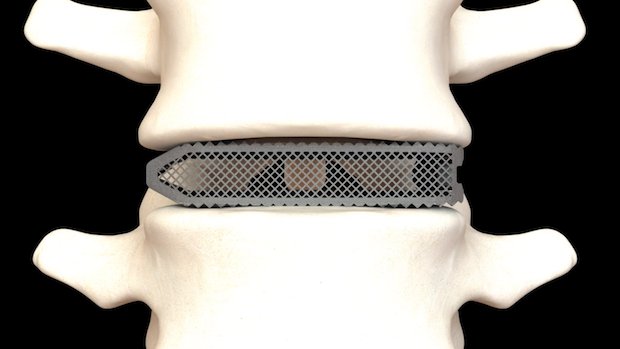
The Aries lumbar interbody fusion devices devices are printed from titanium material, which is optimized for bone fusion and biological fixation. They are 3D printed using Osseus’ proprietary 3D printing technology, PL3XUS, which uses powder bed fusion to build parts in titanium.
Aries devices have a porosity of 80%, allowing spine tissue to grown through the implant. Additionally, the device comes with a mesh for better bone cell fixation and proliferation, allowing for faster healing, improved bone growth and enhanced visibility on x-rays.
The Aries implants are available for lateral, anterior, straight and curved spinal injections. Each Aries device comes in a wide variety of heights and lordotic (inward spinal curvage) angles, adaptable for a variety of patient anatomies.
“The clinical benefits of 3D printed titanium [implants] speak for themselves and Osseus is poised to capture market share in an exponentially growing industry like never before.” said Eric Hansen, co-founder and CEO of Osseus.
A new wave 3D printed implants
Implants for the spine continue to be one of the most common cases of FDA-cleared 3D printed medical devices. Earlier this year, Zimmer Biomet received FDA clearance for the company’s first ever 3D printed titanium spinal implant.
Emerging Implant Technologies also recently received FDA approval for its 3D printed multilevel cervical cage, which can treat multiple injuries in both the middle and top parts of the spine.
Similarly, spinal device development company, Centinel Spine, received FDA clearance for its 3D printed spinal implants, known as FLX devices. The FLX devices are titanium fusion implantates that work to stabilize vertebrae from the front of the spine in order to increase the healing process for patients.
“As a surgeon, it’s very exciting to participate in the device development process and see your ideas brought to life so quickly,” said Dr. Sam Joseph, Jr., who collaborated with the Osseus engineering team. “Using 3D printing, we were able to go from design, to prototype, to finished product much faster than with traditional manufacturing, which means patients get access to more advanced treatments sooner, too.”
Keep up with the latest advancements in additive manufacturing by subscribing to the 3D Printing Industry newsletter. Alternatively, follow us on Twitter, and like us on Facebook.
Featured image is a rendering of an Osseus fusion system. Image via Osseus fusion system,


