Novel materials developer and MIT spin-out OPT Industries has raised $15 million in Series A funding to scale up the development of its novel 3D printing metamaterials and products.
The capital injection will also be funneled into scaling up the production of the firm’s InstaSwab high-efficacy nasal swab developed during the pandemic.
“As an advanced manufacturing company, we see the value in building micro-scale technologies that solve macro-scale challenges,” said Jifei Ou, Founder and CEO of OPT. “OPT works with customers to design and manufacture a novel range of metamaterials and products for the healthcare, automotive, cosmetics and consumer goods industries, and beyond.
“We will leverage the new financing to address the demand for InstaSwab, fuel product development, scale operations, and grow the team.”
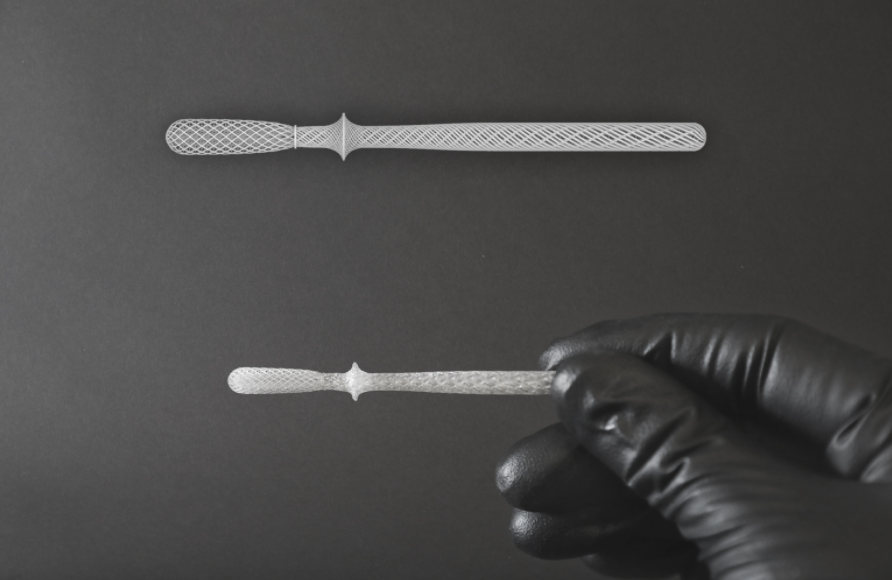
OPT’s roll-to-roll 3D printing platform
OPT Industries emerged as a spin-off company from MIT after seven years of R&D to develop its RAMP roll-to-roll 3D printing platform, which reportedly enables the printing of continuous materials at “unlimited lengths” with micron-level precision.
The RAMP system delivers printing resolutions down to 50µm, and claims to avoid the higher costs and lead times associated with conventional tooling and global supply chains. Alongside its 3D printing platform, OPT also offers its own line of proprietary polymers optimized for micron-scale photolithography 3D printing, and computational design capabilities.
After its establishment as an independent company, OPT rapidly scaled up its technology during the pandemic to design and manufacture its 3D printed InstaSwab nasal swab for Covid-19 testing. The company claims the InstaSwab is up to 20 times more effective in bacterial sample elution than conventionally manufactured nasal swabs, and significantly more effective in viral sample elution.
“In the field of material science, metamaterials is a fast-developing area that holds enormous untapped potential,” said Andrea Jackson, Director at Northpond Ventures, the lead investor in OPT’s Series A funding round. “OPT’s platform is uniquely capable of designing materials with advanced properties and superior performance across a range of applications including absorption, cushion, insulation, and more.
“The InstaSwab – made of precisely engineered fibrous structures, each thinner than a human hair – exemplifies one of the standout innovations in novel metamaterials. OPT’s technology enabled the design of a more sensitive medical diagnostic test, resulting in real-world impact on patient care.”
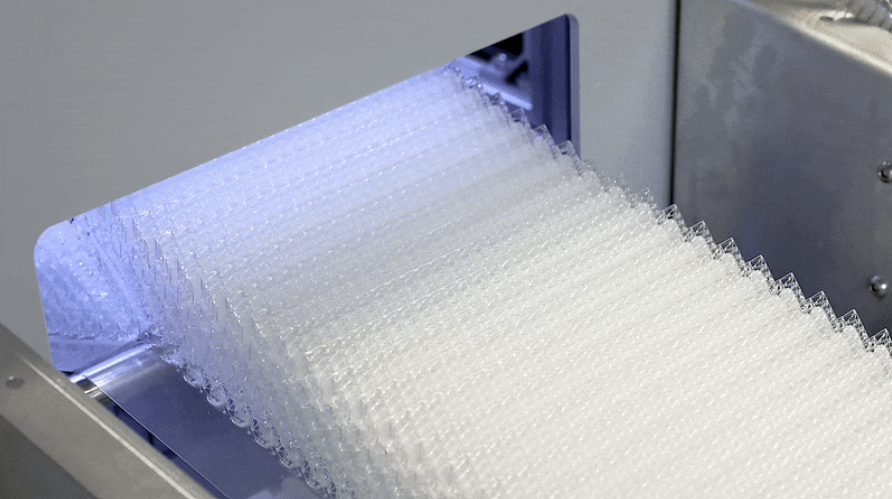
Funding the scale-up of RAMP
Shortly after opening its 14,000 square foot manufacturing facility last year, OPT Industries has now closed a $15 million Series A funding round to continue scaling its operations.
The financing round was led by science-driven venture capital firm Northpond Ventures with participation from existing investors Crosslink Capital and the MIT-affiliated E14 Fund.
OPT will use the funds to address growing demand from its customer base for novel metamaterials design and production, as well as scaling up the production of its InstaSwab. The company will also use the capital injection to fuel its product development activities and to grow its team.
In addition to its financing news, OPT also announced that UK-based care diagnostics company LumiraDx had added the InstaSwab to its list of approved nasal swabs for Covid-19 testing. InstaSwab is reportedly the first 3D printed nasal swab to make the list.
“OPT is a prime example of the next generation of manufacturing and how it will have a material impact on building the supply chain of tomorrow,” said Phil Boyer, Partner at Crosslink Capital. “With technology that can enable rapid iterative design, end-to-end automated manufacturing, and continuous locally produced materials for such a wide range of applications, OPT is fast becoming a critical component for supply chain resilience.”
Going forwards, OPT reportedly plans to continue turning its new 14,000 square foot facility into a state-of-the-art additive manufacturing site capable of producing commercial applications beyond medical devices alone. In fact, the firm says its 3D printing platform and novel materials can be leveraged for various applications across the automotive and consumer goods industries, in addition to its healthcare potential.
“The rapid pace at which OPT has moved its technology from lab to market has been remarkable,” said Habib Haddad, Managing Partner at E14 Fund. “Within months of the company’s spin-out of MIT, OPT quickly found its way across healthcare and testing organizations nationwide.
“Amid growing demand by Fortune 500 countries and market leaders for OPT’s products, the 3D printing system that Jifei has pioneered has unlocked an entirely new paradigm for product creation from inception to manufacturing.”
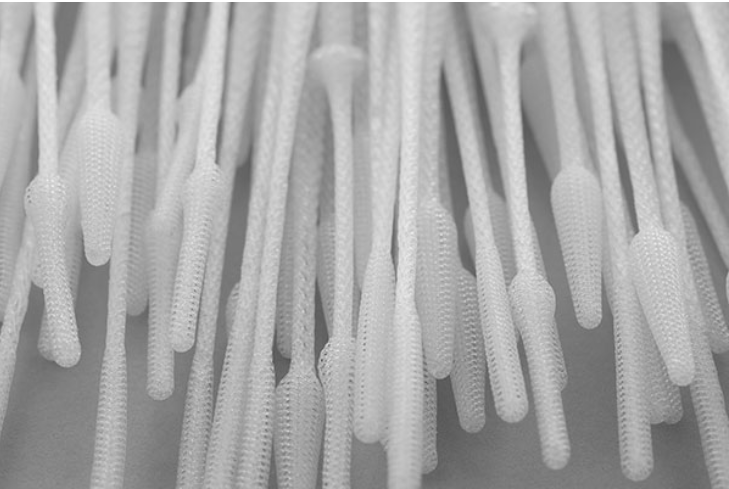
Medical 3D printing investments
Several firms have announced medical-related funding updates and investments in recent months.
For instance, 3D printer manufacturer 3D Systems set out its intentions to expand on its bioprinting program after acquiring Volumetric Biotechnologies for $400 million in October last year. Shortly after, Lightforce Orthodontics raised $50 million in a Series C funding round in order to scale up the production of its custom 3D printed braces and go-to-market efforts.
In December, 3D bioprinting specialist Inventia Life Science announced the closure of a $25 million Series B funding round to ramp up the roll-out of its RASTRUM 3D bioprinter, designed to address the multibillion biomedical R&D and drug discovery market.
Most recently, biotechnology firm Stämm Biotech raised $17 million in Series A funding which it will plow into developing its next-generation 3D printed bioreactor. Cell-based cultures grown in the bioreactor could potentially be leveraged for drug testing purposes and developing medical treatments in the future.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows OPT Industries’ 3D printed InstaSwab nasal swab. Image via OPT Industries.



