Johnson & Johnson Medical GmbH, the subsidiary of Johnson & Johnson Medical Devices Companies (JJMDC) has acquired spinal implant 3D printing specialist Emerging Implant Technologies (EIT), headquartered in Germany.
Following this acquisition, Johnson & Johnson’s orthopedics branch DePuy Synthes will also strengthen its interbody implant portfolio which includes titanium integrated PEEK technology for minimally invasive spinal surgery.
“Our goal is to offer a complete portfolio of interbody solutions that provides surgeons with even more options for the treatment of their patients,” said Aldo Denti, Company Group Chairman of DePuy Synthes. The financial terms of the transaction remain undisclosed.
3D printing at Johnson & Johnson
With over 130 years of experience, Johnson & Johnson is a globally recognizable healthcare company, operating across 57 countries. The company’s overall mission to “profoundly change the trajectory of health for humanity” has prompted various ventures into new technologies such as additive manufacturing.
Most recently, the company collaborated with Trinity College Dublin (TCD), and the AMBER research center to establish a new specialist 3D bioprinting facility for developing personalized healthcare solutions. Prior to this, Johnson & Johnson partnered with Aspect Biosystems to leverage 3D bioprinting to explore treatment common knee injuries. The company has also partnered with Carbon and HP in order to create custom, 3D printed surgical devices.
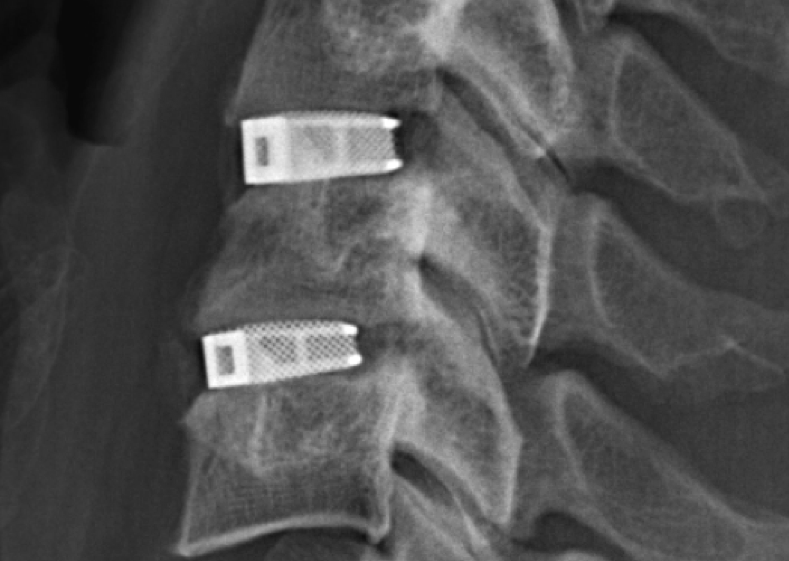
Through a previous deal between Tissue Regeneration Systems and Materialise, Johnson & Johnson subsidiary DePuy Synthes offers the 3D printed titanium TRUMATCH maxillofacial implants for jaw and facial reconstructions, and 3D printed bioresorbable patient-specific implants. Now, with a focus on treating spinal diseases, DePuy Synthes has assimilated EIT’s proprietary advanced cellular titanium 3D printing technology into its portfolio.
Medical 3D printing market consolidation
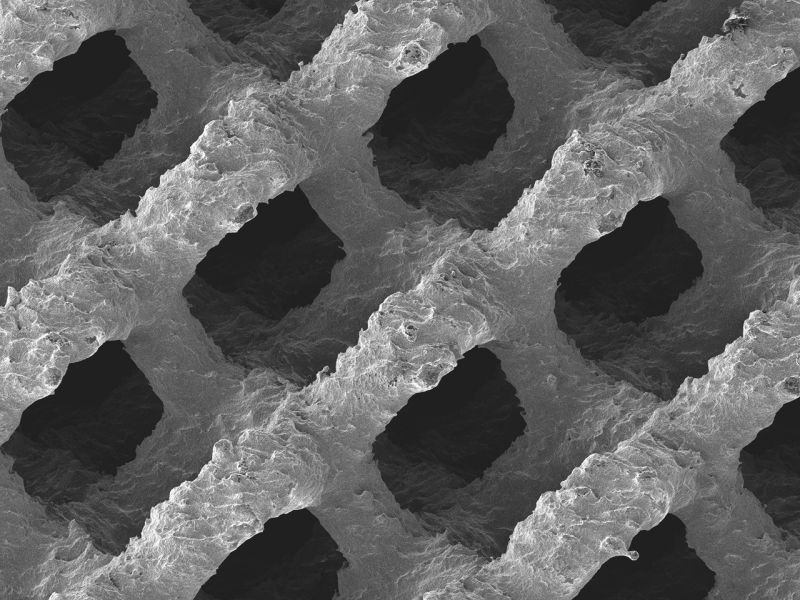
EIT Cellular Titanium implants are made using Selective Laser Melting (SLM), forming open and interconnected porous structures designed to promote bone in-growth. Last year, EIT’s Cellular Titanium implants received 510(k) clearance from the FDA. Prior to this, the implants had been used in 10,000 cases in over 15 countries around the world.
In recent years, there has been a proliferation of 3D printed spinal implants achieving clearance for the commercial market. Now, it seems, the market is experiencing some consolidation. Johnson & Johnson’s EIT acquisition comes just after FORTUNE 500 medical technology firm Stryker acquired Virginia medical device manufacturer K2M in a $1.4 billion deal.
“We are excited to welcome the skilled team at EIT,” concludes DePuy Synthes chairman Denti, “and together, we aspire to bring to market technologies that allow surgeons to perform spinal fusion procedures reliably and with consistent outcomes.”
Keep up with the latest 3D printing news by subscribing to the 3D Printing Industry newsletter. Also, follow us on Twitter, and like us on Facebook.
Searching for new talent or seeking a career change? Search and post 3D Printing Jobs for opportunities and new talent across engineering, marketing, sales and more.
Featured image shows the surface structure of an FDA approved 3D printed cellular titanium implant. Image via EIT.


