Scientists from the Indian Institute of Technology (IIT) Delhi and IIT Kanpur have created a 3D bioprinting method to develop load-bearing bone cartilage.
In a study published in the scientific journal, ACS Biomaterials Science & Engineering, the researchers took inspiration from the biological process within the human body where stem cells form into bones (osteoblasts), to create a 3D printed bone construct.
“A major challenge in bone tissue engineering is to develop clinically conformant load-bearing bone constructs in a patient-specific manner,” the paper states.
“3D bioprinted silk-gelatin constructs enabled adequate cellular attachment, proliferation and most importantly, articular cartilage differentiation for activating pathways of organogenesis [organ production] in a patient-specific manner.”
Forming 3D printed bone constructs
According to the study, load-bearing, long bones, such as the femur, are produced from a process involving stem cells which form cartilage templates. These templates experience further differentiation to form bone cells which are designed to bear weight.
According to Sourabh Ghosh a Professor from the Department of Textile Technology at IIT Delhi, attempts to develop load-bearing bones using different scaffolds have bypassed the template stage of cartilage formation to differentiate stem cells directly into bone cells.
“The efficacy of such bone constructs is yet to be demonstrated in bearing loads. There is a very poor correlation between bone constructs developed in vitro and in vivo. Also, the gene expression pattern of these tissue-engineered bones largely differs from human adult bone.”
Using bioink containing silk proteins, mesenchymal stem cells, a thyroid hormone (Triiodothyronine), and growth factors, as well as a 3D bioprinter, the research team were able to engineer bone cartilage.
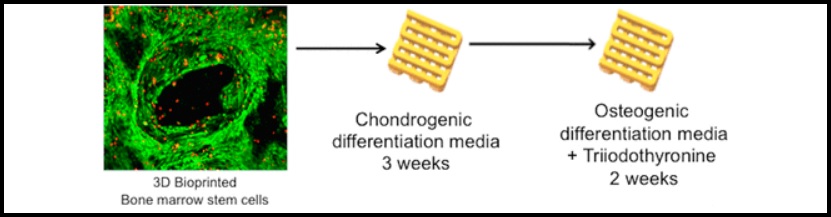
Load-bearing stem cells
Professor Ghosh explained, “We followed a four-step process to develop the load-bearing bone. We first developed chondrocytes (cartilage) from stem cells and then differentiated them into hypertrophic chondrocytes [specialized cells for bone growth].”
“During this process, the sponge-like cartilage becomes a brittle tissue. Here it is following the development biology mechanism to become a bone.”
The hypertrophic chondrocytes then differentiate into osteoblasts prior to them becoming adult bone cells known as osteocytes. Professor Amitabha Bandyopadhyay from the Department of Biological Sciences and Bioengineering at IIT Kanpur further explained:
“Compared to bone formed directly from stem cells, the extracellular matrix of the bone construct developed through the intermediate cartilage process was 10s of times higher.”
“The load-bearing capacity of a bone depends primarily on the quality of extracellular matrix. In loading-bearing bones, the extracellular matrix comprises 95% while bone cells are just 5%. So if you are trying to fabricate a load-bearing bone construct it is better to have more extracellular matrix.”
The researchers have deduced that the bone construct demonstrated better results than the bone developed directly from stem cells. The team intends to undertake studies on animals.
The research paper, “Developmental Biology-Inspired Strategies To Engineer 3D Bioprinted Bone Construct” is co-authored by Shikha Chawla, Aarushi Sharma, Amitabha Bandyopadhyay, and Sourabh Ghosh.
Submit your nominations for the 2019 3D Printing Industry Awards here.
Also, for all the latest 3D printing news, subscribe to the 3D Printing Industry newsletter, follow us on Twitter, and like us on Facebook.
Make your next additive manufacturing career move or hire new talent. Search and post 3D Printing Jobs on our free jobs service.
Featured image shows an X-ray of cartilage damage in the knee. Image via medicalnewstoday,


