Glass, electronics, chemicals, and ceramics manufacturer AGC Inc. has developed a biocompatible non-cytotoxic urethane acrylate oligomer that can be 3D printed into ultra-realistic patient-specific organ models.
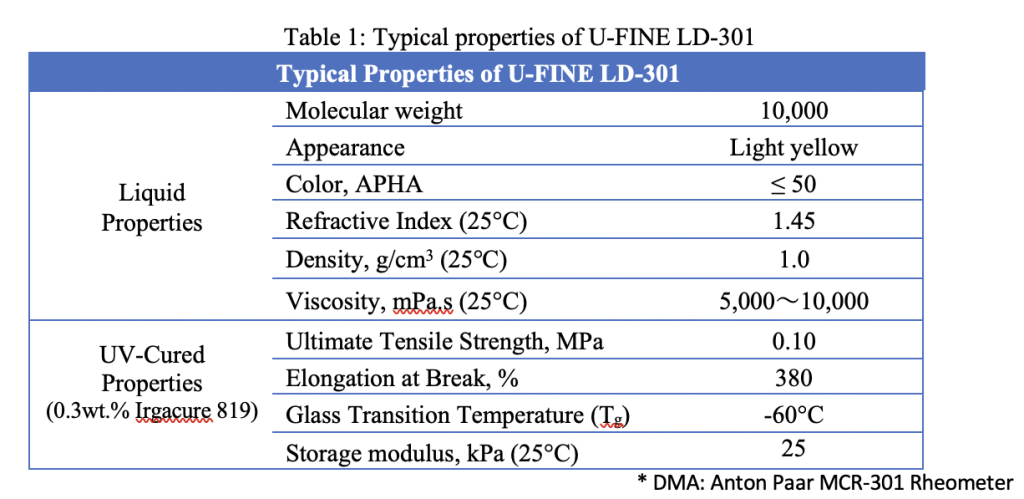
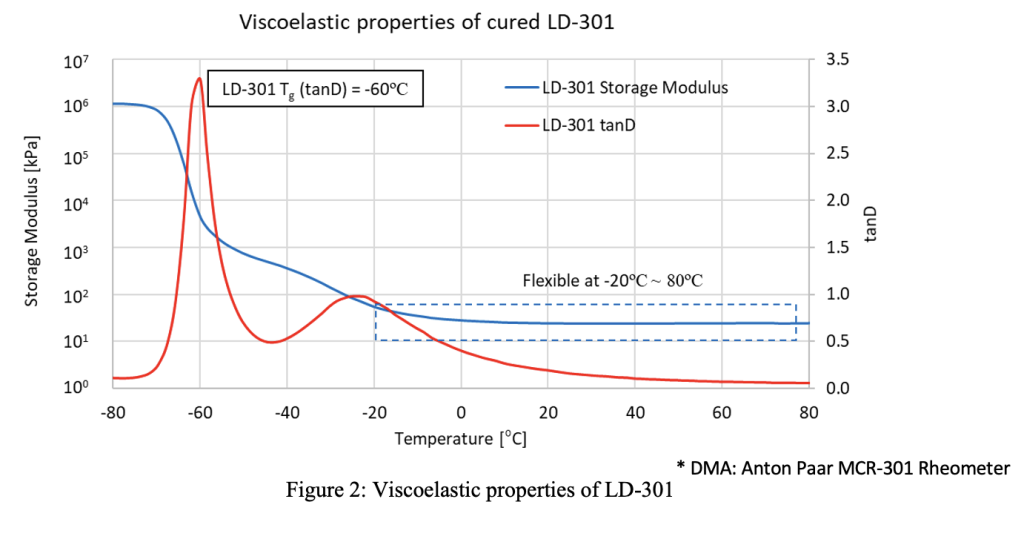
With its new oligomer, AGC Inc. has managed to design a material that features a low storage modulus without using plasticizers. As a result, ‘U-FINE LD-301’ can be used as a basis for non-cytotoxic stereolithography (SLA) or Digital Light Processing (DLP) 3D printing resins that are less susceptible to dimensional instability or bleeding issues.
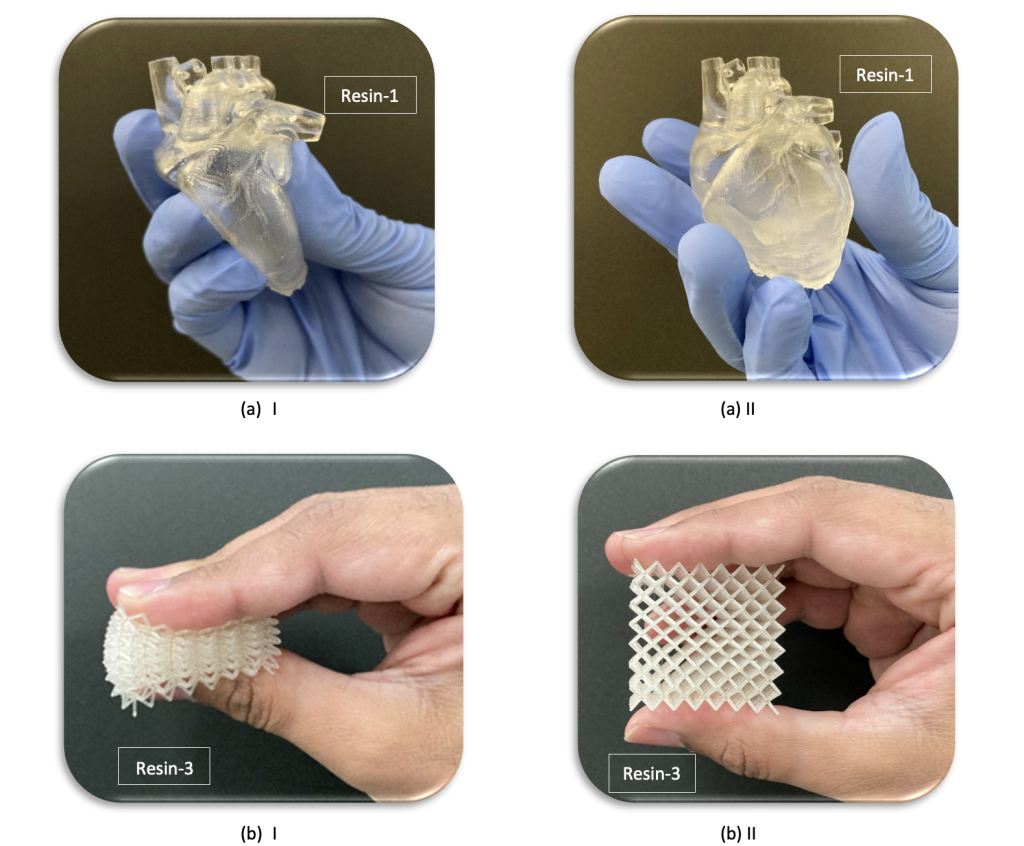
In practice, the firm expects its stretchable new material to be adopted among the medical community as a means of 3D printing incredibly lifelike, patient-specific anatomical models, with wide-ranging clinical training applications.
“We have designed this material specifically for organ modeling,” explains Nikhil Mishra, a Product Development Engineer at AGC Inc. “So, which of its properties lend themselves towards this application? It is biocompatible, ISO-10993:5 approved. It also has a low storage modulus, glass transition temperature and shrinkage rate.”
A new SLA/DLP resin base material
Though the additive manufacturing of patient-specific clinical models is by no means unheard of in the medical profession, and there are plenty of use cases to support this, the process still has its challenges. To improve their softness, many resins include plasticizers that impact both their biocompatibility and dimensional stability.
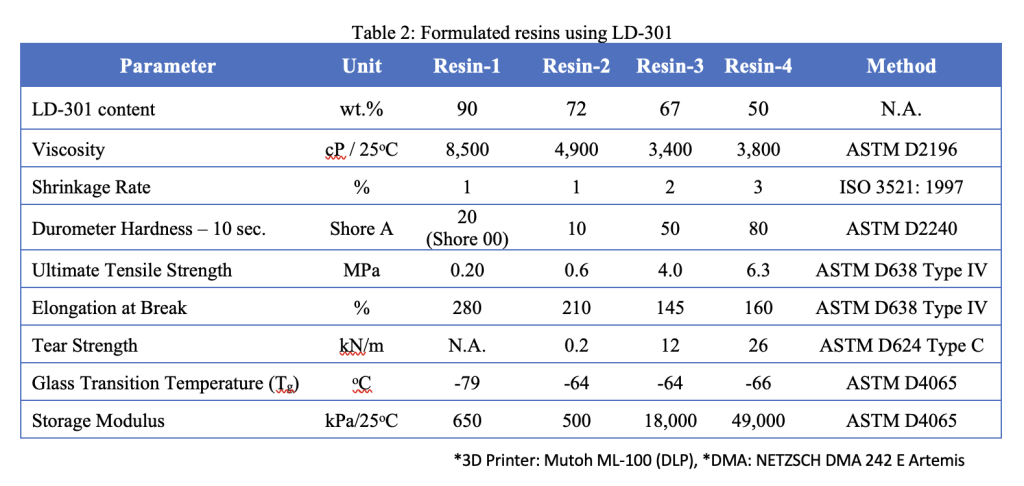
To get around these issues, AGC Inc. has come up with a new material formulation, which it has managed to make plasticizer-free by focusing on a few integral properties: elastic modulus, glass transition temperature (Tg), shrinkage rate, and viscosity.
The company chose to focus on developing a material with a tunable stiffness due to each organ’s differing elastic modulus. Soft organs like kidneys, hearts, and intestines, for instance, have an elastic modulus of around 10 ~ 40 kPa. In order to 3D print an accurate model, it’s therefore essential to have a resin that enables the creation of good interlayer joints and offers high dimensional stability.
AGC Inc. has also found that minimizing resin shrinkage and viscosity is vital to ensuring print accuracy while reducing any risk of brittleness. As a result, LD-301 is designed to yield resins with a low viscosity, stiffness and storage modulus.
Targeting clinical model 3D printing
According to AGC Inc, LD-301’s novelty lies in its high molecular weight, as well as its mono functionality, low cross-linking and biocompatibility properties, which make it ideal for formulating biocompatible DLP and SLA resins. In fact, during ISO cytotoxicity testing, a cured version of the company’s material is said to have achieved higher than 70% cell viability, and it was found to have no significant impact on the formation of cell colonies.
In terms of elongation at break, cured LD-301 also demonstrated a high elongation of 380%. What’s more, oligomers tend to feature high viscosity, but due to the material’s unique molecular structure, which sees it incorporate monomers as diluents, it has lower viscosity, in a way that makes it a better-suited base for printable resins.
Together, AGC Inc. says these properties mean the oligomer is ideal for creating resins that can be turned into realistic anatomical models, with surgical education and planning applications. As the material has shown the capability to resist repeated compressions and return to its initial shape, it’s believed that LD-301 could be used for insoles as well, though it’s primarily being marketed as a medical modeling tool.
Those interested in finding out more about the material or placing an order can contact AGC Inc. directly for a quote.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? featuring discussion, debriefs, video shorts and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows a set of prototype anatomical models and lattice parts 3D printed from U-FINE LD-301. Image via AGC Inc.



