3D printer manufacturer 3D Systems has added a new hybrid maxillofacial surgical guide to its Virtual Surgical Planning (VSP) medical planning portfolio.
Combining the strength of titanium with the softness of nylon, 3D Systems’ surgical tool allows for occlusal registration, which enables surgeons to operate with greater precision and confidence. Developed in response to client feedback, the guides are already being deployed at the New York Center for Orthognathics and Maxillofacial Surgery, to provide medics with a means of optimizing patient outcomes.
“The new VSP Hybrid Guides are the next generation of occlusal-based surgical guides,” said Dr. Jay Neugarten, an Oral Surgeon at the Center. “To be able to combine two materials – nylon and titanium – to create cutting and predictive hole-drilling surgical guides that register to the dentition, enables a precise and accurate surgical outcome for my patients.”
“VSP surgical planning and guides are an integral part of my ability to deliver highly successful outcomes for my patients undergoing corrective jaw surgery.”
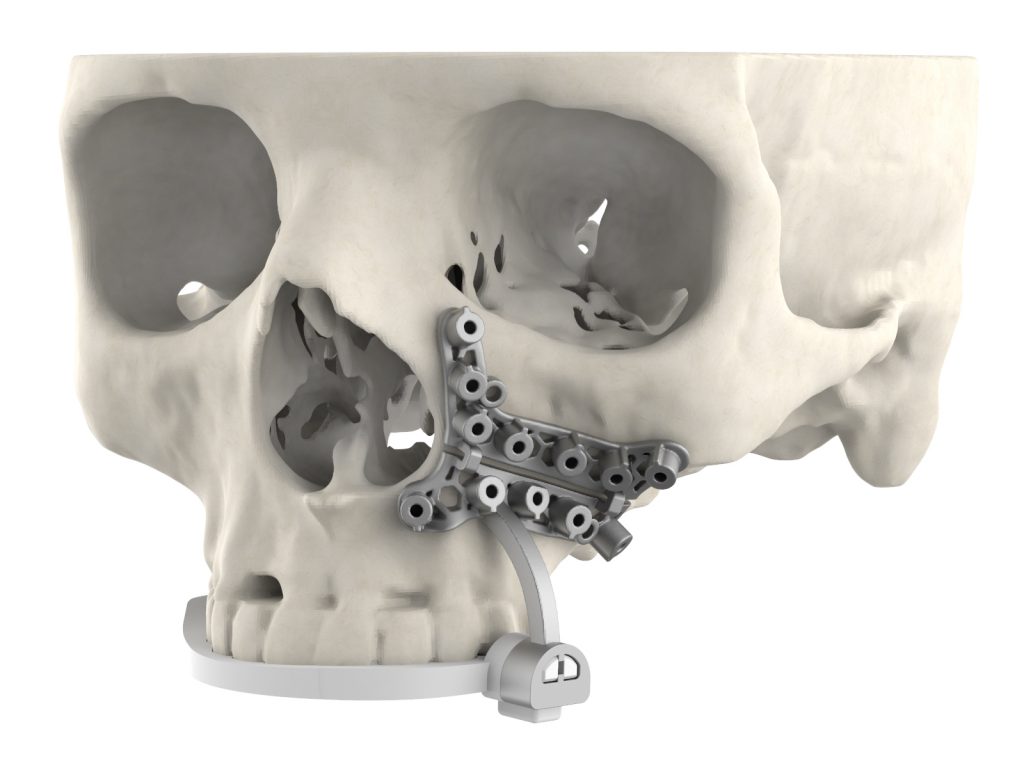
3D Systems’ VSP portfolio
Over the last year, 3D Systems has undergone a ‘strategic resizing’ which has seen it refocus on its core medical and industrial verticals, and offload its Battery Ventures and Cimatron subsidiaries. In that time, the company has significantly expanded upon its medical portfolio, achieving a “breakthrough” in 3D bioprinting alongside United Therapeutics, and producing over a million surgical implants.
Elsewhere, 3D Systems’ VSP service, which combines imaging, simulation, and 3D printing to create a fully-fledged surgical plan, is rapidly becoming central to its clinical offering. The company’s anatomical solutions have already been deployed in more than 140,000 patient cases, while its range of FDA-approved implants continues to grow.
In September last year, the firm’s VSP Orthopaedics platform, which is marketed via Onkos Surgical’s My3D service, attained 510(k) clearance from the FDA. Not long after, 3D Systems’ also gained FDA approval to 3D print maxillofacial surgical guides using its LaserForm Ti and DuraForm ProX PA materials, which have now entered a limited launch via its medical partner Stryker ahead of a full May 2021 release.
“With the introduction of VSP Hybrid Guides, we’ve elevated the capability of our renowned VSP surgical solutions portfolio to facilitate craniomaxillofacial procedures,” said Menno Ellis, Executive VP of Healthcare Solutions at 3D Systems. “In partnership with our expert biomedical engineers, surgeons can develop effective surgical plans and patient-specific devices that help improve patient outcomes.”
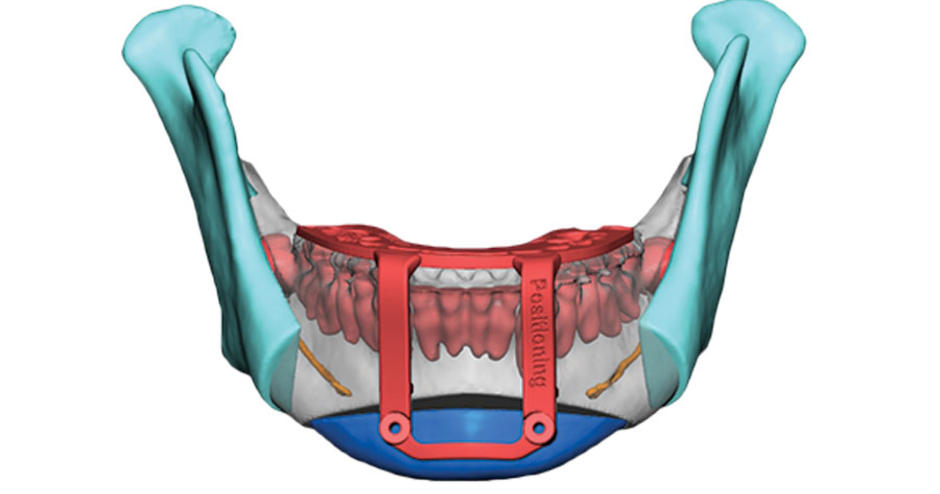
Streamlining cranial surgery
Designed to help surgeons deliver better outcomes for their maxillofacial patients, 3D Systems’ latest medical device incorporates nylon to enable tooth-based registration. As a result, the new tool can effectively be placed by clinical users with greater confidence than conventional devices allow, yielding enhanced surgical site visibility.
What’s more, occlusal registration potentially leaves patients with a reduced guide footprint, and the device’s slim overall profile means that it can be accurately placed in hard to reach areas. Produced using the firm’s LaserForm Ti and DuraForm ProX PA materials, the new guides are also easy to assemble, and can even be customized to embed multiple cutting/drilling locations onto a single device.
When combined with 3D Systems’ wider VSP offering, Neugarten says that the VSP Hybrid Guide offers the precision levels of an automotive sat nav system, serving to make the surgical process more efficient and potentially saving both surgeons and patients hours in the operating theater.
“Using the VSP Hybrid Guides together with Stryker’s Facial iD customized plates streamlines my workflow in surgery,” concluded Neugarten. “This ability to seamlessly transfer my digital treatment plans to the surgical arena allows me to provide the highest level of care for my patients.”

Facial reconstruction advances
In addition to producing guides that enhance patient outcomes, 3D printing is now being used on a more experimental basis, to create facial grafts that can be implanted into patients’ skulls. Just last year, for instance, scientists at Texas A&M University developed a novel additive manufactured scaffold that directly facilitates bone cell growth after surgery.
Within more end-use applications, the Québec Industrial Research Centre (CRIQ) has adopted a GE Additive Arcam Q10 3D printer in order to expedite its production of patient-specific lower jawbone implants. The organization switched to additive manufacturing, with the aim of reducing the devices’ lead times from around six weeks down to just three.
Researchers at Paulista University, meanwhile, have taken patient-specific implants a step further, by 3D printing a facial prosthetic for a Brazilian cancer survivor, which included her entire right eye. Using images captured from a smartphone, the team were able to create the implant less invasively than conventional grafts allow.
More information about the VSP Hybrid surgical guide can be found in the paper titled “Computer-aided stereolithography for presurgical planning in fibula free tissue reconstruction of the mandible.” The research was co-authored by Jill Sink, David Hamlar, Deepak Kademani and Samir S Khariwala.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows 3D Systems’ new 3D printed surgical guide attached to a skull. Image via 3D Systems.



