3D printed patient-specific airway stents, developed by a doctor at Cleveland Clinic, a nonprofit multispecialty academic medical center in Ohio, has received clearance from the U.S. Food and Drug Administration (FDA).
Created by Cleveland Clinic physician Tom Gildea, M.D., the 3D printed silicone stents are used to help patients with serious breathing disorders by keeping their airways open. Such breathing disorders can be caused by conditions such as tumors, inflammation, trauma or other masses. Prior to approval from the FDA, the 3D printed stents were being implanted within patients under the compassionate use program, which allows patients who have failed all available forms of treatment to receive investigational ones not yet available to the public.
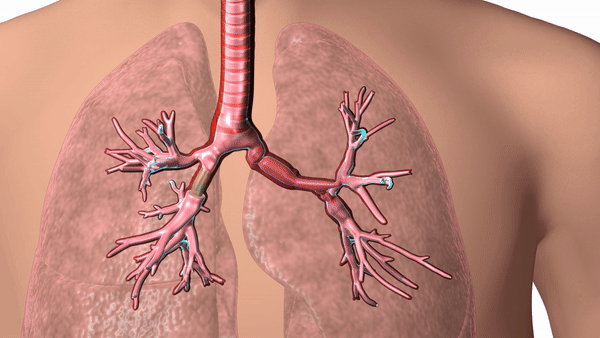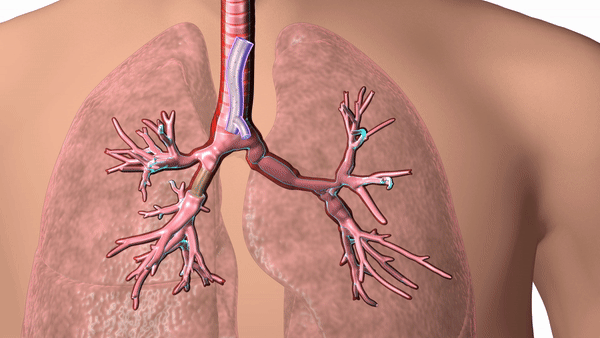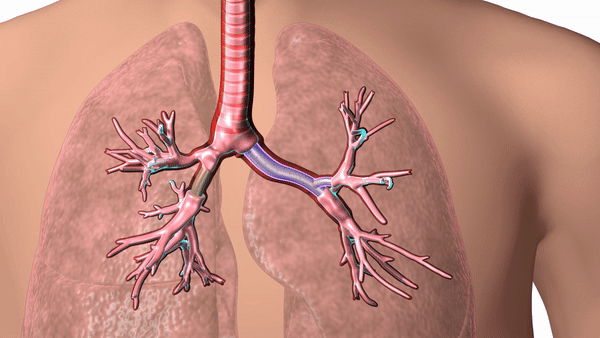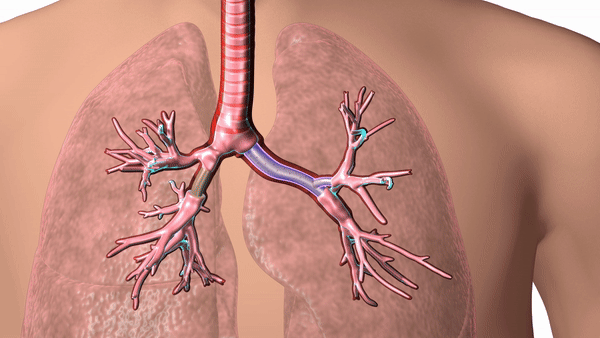
The stents are designed according to patient-specific data gathered using CT scans and proprietary 3D visualization software and have helped to overcome problems surrounding ill-fitting standard stents, which can cause airway complications in patients.
“Breathing is something many people take for granted, but for many of these patients, every breath can be a struggle. It’s been gratifying to see patients receiving the customized stents feeling relief right away,” comments Dr. Gildea, section head of bronchoscopy at Cleveland Clinic.
“We are excited to be able to bring this technology to more patients across the country and grateful for the patients and donors who have worked with us to help pioneer this technology.”

The advantages of 3D printed airway stents
It’s estimated that about 30,000 airway stents will be implanted in the U.S. in 2020. Standard airway stents are designed with a limited number of sizes and shapes and are most often suitable for individuals with larger airways. This narrow scope for customizability makes it difficult when it comes to implanting the stents within patients, as no two patient anatomies are alike, making it difficult to get a perfect fit, especially for those with complex conditions. Stents that aren’t fitted properly within patients can kink and bend, and they can also cause airway complications such as the growth of new tissue, mucous impaction, and tissue death.
In response, Dr. Gildea came up with the idea of 3D printing the airway stents according to patient-specific data, enabling a personalized fit for each patient’s anatomy. Using CT scans and proprietary 3D visualization software, Dr. Gildea and his engineering team design the stents, and then 3D print the molds according to the design. The molds are then injected with medical-grade silicone.
As well as enabling a perfect fit, the use of patient-specific silicone stents also provides the potential advantage of being more tolerable than traditional silicone stents. Stock stents may have to be frequently changed or cleaned due to problems from a poor fit in certain patients.
However, studies have shown that patient-specific stents lasted a year on average, compared to 90 days for standard airway stents. Additionally, patient-specific stents have demonstrated shorter procedure times and improved patient-reported symptoms. This can prove beneficial as it reduces the need for stent changes and modifications, a common problem with the standard counterpart.
Providing patient-specific products for a personalized healthcare experience
Patient-specific products manufactured using 3D printing provides many benefits for the medical sector as a whole. As well as providing a personalized experience for patients, patient-specific products can also better inform medical professionals and prepare them for operations. It is, therefore, an area in constant development as hospitals continue to adopt 3D printing technologies to provide personalized healthcare. For example, The Veterans Affairs (VA) Puget Sound Health Care System recently announced a two-year partnership with the University of Washington School (UW) of Medicine to create patient-specific 3D printed models to help treat mitral valve disease, a complex heart abnormality. Recently, GE Healthcare and Formlabs announced a collaboration aiming to make it easier for clinicians to 3D print patient-specific anatomical models from imaging data.
As such, 3D printed patient-specific products, including the airway stents, were named as one of the top 10 innovations at Cleveland Clinic’s annual Medical Innovations Summit in 2018. Dr. Gildea was also the recipient of the Outstanding Innovation in Medical Device award at the 2018 annual Inventor Awards Reception held by Cleveland Clinic Innovations.
As personalized medical devices commonly arrive in the form of orthopedics, an orthopedic-focused Cleveland Clinic subsidiary was behind the development and FDA clearance of the 3D printed patient-specific stent. To manufacture and provide the 3D printed stents, a new subsidiary named VisionAir Solutions will be formed, which will also work on bringing other 3D printed devices to interventional pulmonologists and the patients who need them. By the end of 2020, VisionAir Solutions plans to begin providing the personalized stents to patients in a controlled launch at medical institutions around the country.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows a diagram of 3D printed airway stent in place. Image via Cleveland Clinic.


