University of California, Berkeley (UC Berkeley) researchers have developed a freezing-modulated cross-linking approach that could allow for the 3D bioprinting of human tissues with improved clinical viability.
Unlike ordinarily-bioprinted tissues, which can be too soft to support their own weight, the team’s technology sees bio-inks frozen and cross-linked, facilitating the creation of cell structures capable of retaining a desired shape. Using this ‘3D cryoprinting’ process, the scientists believe it may be possible to produce robust alginate scaffolds seeded with cells, either for lab culturing or tissue regeneration.

Rethinking the ‘3D cryoprinting’ process
Although 3D bioprinting has become a popular means of engaging in cell-based research, the technology remains difficult to use with softer biomaterials like sodium alginate. Despite the seaweed-based complex carbohydrate’s relative affordability and strong biocompatibility, it continues to come with lengthy lead times caused by necessary cross-linking.
In order to get around the drawbacks of such bio-inks, many researchers now print them into structures layer-by-layer using 3D cryoprinting, a process that sees them rapidly cooled and frozen into place upon deposition. However, as the Californian team point out in their paper, the technology’s approach to solidifying materials also makes them difficult to cross-link, and thawing can lead to their collapse.
To make matters worse, the scientists add that 3D cryoprinting often necessitates the use of internal crosslinking, which only allows bio-inks to be extruded for short periods before they suffer from high viscosity, and the process of defrosting also reduces scaffold viability, particularly in tissue engineering applications.
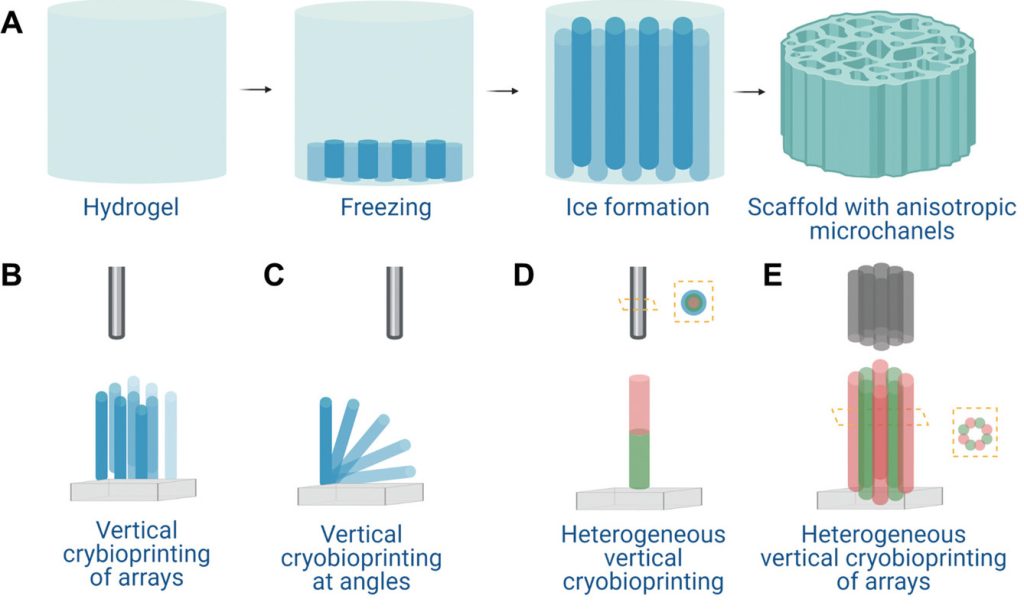
Honing a freezing-modulated alternative
In their efforts to come up with an alternative to conventional 3D cryoprinting, the scientists have developed freezing-modulated cross-linking, a process in which structures are frozen but then thawed in a calcium bath. Through doing so at a controlled rate, the team says it’s possible to cross-link tissues layer-by-layer, thus allowing them to retain a set shape.
During the early stages of their technology’s R&D, the researchers began by using a mathematical model to identify the optimal temperature at which materials should be frozen and defrosted. Once these parameters had been established, the team harnessed their process to produce a variety of multi-layer alginate objects, which could be refrigerated or kept frozen for later cell-based experimentation.
Initial results showed the quantity of calcium that materials were bathed in, had no impact on their shrinkage, and lowering the process’ temperature wasn’t found to yield any accuracy benefits either. That said, when it came to defrosting the scaffolds, melting these at a higher rate did cause them to shrink, with tissues cross-linked at 20 °C becoming smaller than those cross-linked at -0.05 °C.
Having identified cross-linking at an object temperature of -80 °C and bath temperature of -0.05 °C, as the ideal parameters for their process, the researchers say it could now widen 3D cryoprinting’s applications. However, the team also accepts that further research is needed into the way their approach affects cell viability, something that’ll be critical to its deployment in regenerative tissue R&D.
Freezing in 3D bioprinting development
As is the case with traditional 3D bioprinting, cryoprinting continues to be the subject of intense lab-based research, with scientists around the world seeking to perfect and experiment with the technology. Earlier this year, a team at Harvard Medical School and Sichuan University developed a novel means of 3D printing freestanding, mixed-cell tissues, including live human muscle-tendons.
Back in 2018, researchers at Imperial College London, also developed a similar method, which involved using cryogenics to 3D print soft tissues, capable of fooling the human lungs and brain into thinking they’re genuine. At the time, the seeding of live dermal fibroblast cells onto 3D printed scaffolds was seen as an important step forward that showcased the potential of cryoprinted inks.
Elsewhere, other 3D bioprinting developments have seen the technology advanced to the point that it can be used to produce highly-complex cell structures. Late last year, for instance, scientists at the University of Montréal, Concordia University and the Federal University of Santa Catarina, were able to 3D bioprint mice brain cells.
The researchers’ findings are detailed in their paper titled “Freezing-modulated-crosslinking: A crosslinking approach for 3D cryoprinting,” which was co-authored by Linnea Warburton and Boris Rubinsky.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
For a deeper dive into additive manufacturing, you can now subscribe to our Youtube channel, featuring discussion, debriefs, and shots of 3D printing in-action.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows UC Berkeley’s Etcheverry Hall facility, which houses its mechanical engineering department. Photo via UC Berkeley.



