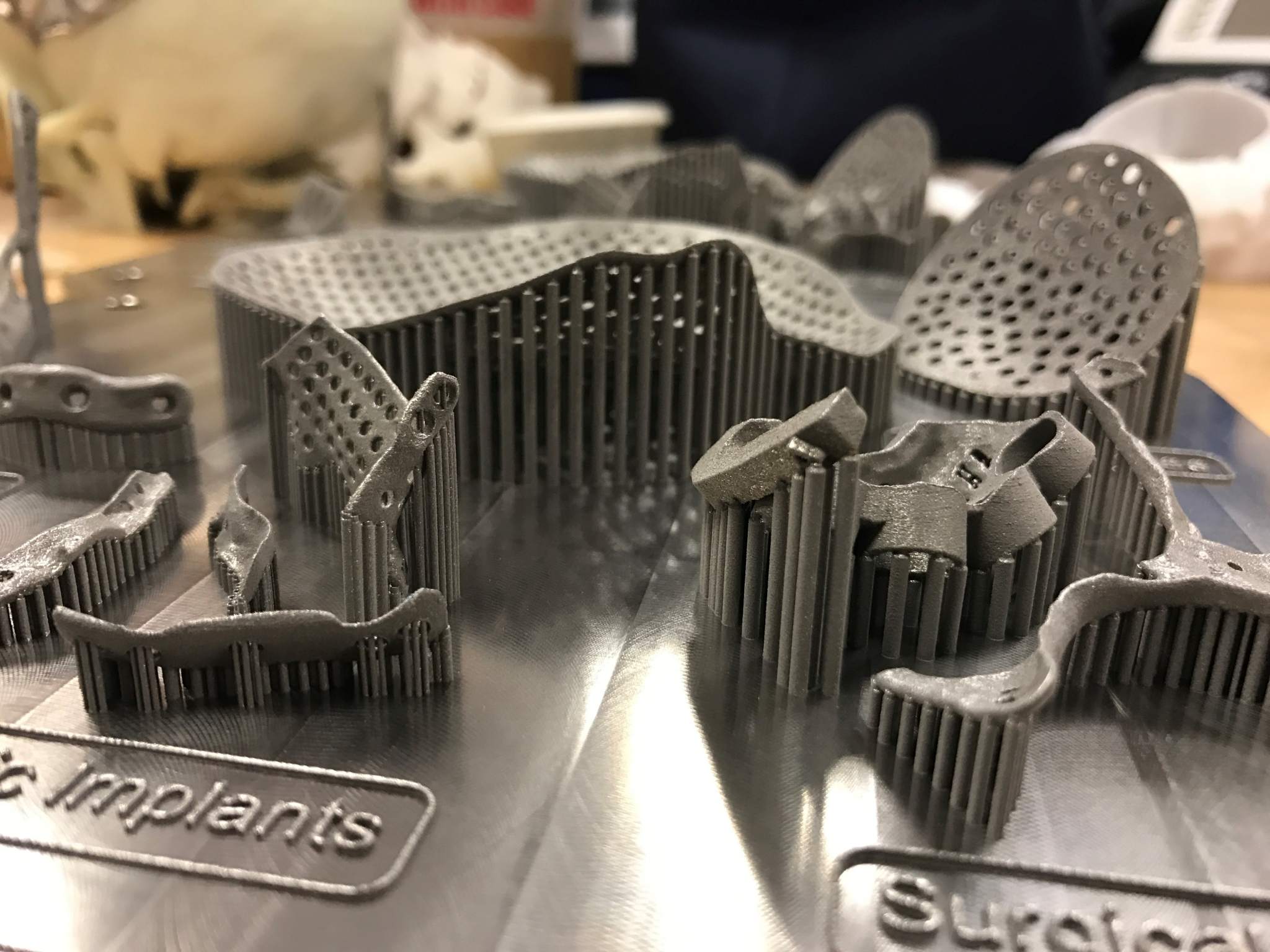
British manufacturing company Renishaw is supporting the establishment of a specialist medical 3D printing center in Southern Ontario, Canada. Known as the Additive Design in Surgical Solutions (ADEISS) Centre, the project is the result of a partnership with the London Medical Network (LMN), Renishaw, and Western University (WU).
ADEISS aims to provide London, Ontario with a leading centre for the production of metal additive manufactured medical tools and implants with the project funded by a $5 million USD ($6.8 million CAD) investment.

The three organizations will all provide different solutions for ADEISS, with Renishaw supplying the additive manufacturing technology. The London Medical Network will bring their existing expertise in the medical supplies industry and Western University will spearhead research and development. The ADEISS center will be located at Western University’s Discovery Park.
This collaboration marks Renishaw’s second North American additive manufacturing initiative in recent months, having just announced the opening of an additive Solutions Center in nearby Kitchener, Ontario.
The vision of ADEISS
Paul Paolatto, CEO of ADEISS explains how the company aims to operate, “someone takes a view of a knee, or a hip or a cranial plate, ships it to us and we make it and ship it overnight for installation the next day. That is the vision.” 3D printed implants are becoming more and more popular for use in the medical industry as they provide an unparalleled service for custom made parts. An Australian woman recently had her jaw reconstructed with a 3D printed titanium implant.
Initiating sales
ADEISS are currently “pursuing ISO 13485 accreditation and certifications in the United States, Canada and Europe.” The group stating that they expect, “to have secured the appropriate approvals necessary to initiate sales of its products and processes by January 1st, 2018.”
Securing the appropriate certification is key to the application of additive manufacturing in tightly regulated sectors, such as healthcare or aerospace. Fulfilling these strict criteria, and the experience gained in doing so, is seen as core to the strategy of a number of the major OEMs and also more recent market entrants. As 3D Printing Industry recently reported German prosthesis producers Mecuris gained CE certification for their 3D printed foot prosthetics.
“The future of innovation”
Amit Chakma, Western University President, explains how partnerships like this – “which bring together industry, clinicians, researchers and academia, are the future of innovation.” However, there are similar partnerships which are innovating in the present with the Southern Ontario Network for Advanced Manufacturing and Innovation (SONAMI) also announced in this region of Canada. The SONAMI project brings together a number of academic institutions in the hope of creating jobs in additive manufacturing.
If you haven’t already, remember to cast your vote in the 3D Printing Industry Awards ahead of the event next month.
For the latest 3D printing news, sign up to our newsletter and follow us on twitter.
Featured image shows the WU campus in London. Photo via Western University.



